{TL;DR}
Q: Should I take any of the current vaccines against SARS-CoV-2/SARS-2/Chinese CCP Coronavirus/Wuhan Plague/Kung Flu/COVID-19?
A: Only if you feel like being a guinea pig.
Q: If I'm pressured into taking one, which one would be the safest?
A: Ranking by known "safety"
[As safe as it can get for a vaccine]
BCG vaccine = Polio vaccine = MMR vaccine = Tetanus vaccine = Diphtheria vaccine
[Safety among types you should not take]
Novavax and other protein subunit vaccines that just deliver the spike+adjuvant > conjugated vaccines
[Safety among types you seriously should not take]
J&J, Sputnik and other human adenovirus vector vaccines > inactivated virus vaccines > replicating viral vectors (these may include "bad" batches of non-replicating vector vaccines) > Astrazeneca and other non-human adenovirus-vectored vaccines ~ other non-replicating viral vectors ~ pfizer/moderna/any mRNA payload vaccines > any chinese vaccines > live attenuated virus vaccines
Q: I just need to convince a relative into not taking it, is there anything easily digestible and without the difficult technical details?
A: There is:
1) One .pdf from an anonymous Canadian
https://pdfhost.io/v/JlwLHwnhU_COVID19_Leaky_Vaccine_Hesitancy.pdf
2) Another .pdf, a bit long, and beware - it uses fake virus stats there and there
https://aim4truth.org/wp-content/uploads/2021/04/Front-Line-Workers-Testimony-on-Vaccinations.pdf
{Not TL;DR}
*******************************************************************************************
Table of contents (CTRL+F the line to go to the corresponding section for details and sources)
>[Minimizing and mitigating the possible side effects or health damages from the vaccines]
[Actual vaccine efficacy based on data from samples with proper size?]
[When RCT are not RCT: Long-Term Studies Of SARS-2 Vaccines Hurt By Placebo Recipients Getting Immunized]
[Natural immunity is more effective than the vaccine immunity]
[Breakthrough infections?]
[List of current vaccines?]
[Side-effects? Adverse events? Complications? Differences in pathology?]
[Some of the reported adverse events (including the ones where, supposedly, placebo or anything else were "reported" to be the cause)]
[Studies on adverse events]
[Vaccine adverse event trackers]
["Underreporting" is one of the main limitations of VAERS and other similar voluntary adverse event reporting systems]
[Efficacy of vaccines by type?]
[Pathogen priming / autoimmune complications]
[Will I still be spreading the virus after being inoculated?]
[For how long does the immunity from vaccines last?]
[Is vaccines' efficacy affected by virus mutating?]
[Vaccine shedding? S protein shedding? Shedding of vaccine-resistant strains upon infection?]
[Enhanced Respiratory Disease (ERD)? Antibody-Dependent Enhancement (ADE)?]
[Available info on ADE with SARS-CoV-2]
[At least 2 distinct types of ADE]
[Original Antigenic Sin (OAS)?]
[Immune system reprogramming?]
>[Any additional detailed, sourced info?]
[On Marek's disease]
[Human adenovirus-vectored vaccines may be of limited use in the long term, as the human body develops immunity against the adenovirus]
[Pfizer leak] [!]
>[If you want to be vaccinated with something with known risks, safety, and at least some actual efficacy, then consider getting a BCG shot and/or a polio shot and/or an MMR shot and/or a tetanus shot and/or a diphteria shot instead]
>Supplementary food for thought
[You're considered to be not fully vaccinated within first two weeks after your latest SARS-2-vaccination]
[C.D.C. Will Not Investigate Mild Infections in Vaccinated Americans]
[At least in USA, "breakthrough", aka "cases of infection within the vaccinated individuals" are being "counted" via a criteria that would allow for only anomalous and extremely high-viral-load cases to be counted as positive]
[In order to encourage American workers to get vaccinated, the Occupational Safety and Health Administration has suspended the legal requirement for employers to report work-related injuries resulting from SARS-2 vaccinations]
[Australia - Collating vaccination status of cases is not NSW's priority, says CHO]
[Pfizer chief says people will probably require yearly SARS-CoV-2 vaccine booster shot]
[Reanalysis of the Pfizer mRNA BNT162b2 SARS-CoV-2 vaccine data fails to find any increased efficacy following the boost: Implications for vaccination policy and our understanding of the mode of action]
[Pharmaceutical corporations given vaccine liability exemptions from governments]
[Governments' and pharmaceutical corporations' vaccine agreement leaks]
[Developers of Oxford-AstraZeneca Vaccine Tied to UK Eugenics Movement]
[Oxford Kept SARS-2 Vaccine Trial Volunteers in Dark About Dosing Error, Letter Shows]
[3 of the 4 most known SARS-CoV-2 vaccine makers have been sued for products they brought to market even though they knew injuries and deaths would result.]
[Trial dates of the vaccines distributed for emergency]
[As of September 2021, The Pfizer vaccine is still extended under the EUA Emergency Use Authorization]
[Funding, shareholders and connections]
*******************************************************************************************
>[Minimizing and mitigating the possible side effects or health damages from the vaccines]
Due to basic and obvious similarities between virus and vaccines' cellular processes and pathology, if you are interested, refer to:
For starters, use CTRL+F [Pre-vaccination measures] in there, and use the rest of the information on page as if you're to deal with an infection.
[Actual vaccine efficacy based on data from samples with proper size?]
Unknown, mRNA ones unsurprisingly seem to be LESS effective than natural immunity, you may assume the other designated SARS-CoV-2 vaccine types are around as ineffective
>The estimated vaccine effectiveness in preventing infection >7 days after second dose was 86% (95% CI 72-94%)
>Having a prior positive test was associated with 91% (95% CI 85 to 94%) effectiveness against new infection among the unvaccinated
https://www.medrxiv.org/content/10.1101/2021.04.20.21254636v1
>The average half-life of neutralizing activity in the vaccinees was approximately 67.8 days and the average time length for their serums to lose the detectable neutralizing activity was 198.3 days.
https://www.medrxiv.org/content/10.1101/2021.07.27.21261237v1
[When RCT are not RCT: Long-Term Studies Of SARS-2 Vaccines Hurt By Placebo Recipients Getting Immunized]
https://archive.is/a4Gfo
[Natural immunity is more effective than the vaccine immunity]
>The estimated vaccine effectiveness in preventing infection >7 days after second dose was 86% (95% CI 72-94%)
>Having a prior positive test was associated with 91% (95% CI 85 to 94%) effectiveness against new infection among the unvaccinated
https://www.medrxiv.org/content/10.1101/2021.04.20.21254636v1
>Our results question the need to vaccinate previously-infected individuals.
https://www.medrxiv.org/content/10.1101/2021.04.20.21255670v1
[]
[FDA data suggest that vaccines may be even worse in efficacy than placebo]
>Suspected COVID-19 cases that occurred within 7 days after any vaccination were 409 in the vaccine group vs. 287 in the placebo group. It is possible that the imbalance in suspected COVID-19 cases occurring in the 7 days postvaccination represents vaccine reactogenicity with symptoms that overlap with those of COVID-19.
https://www.fda.gov/media/144245/download#page=42
[]
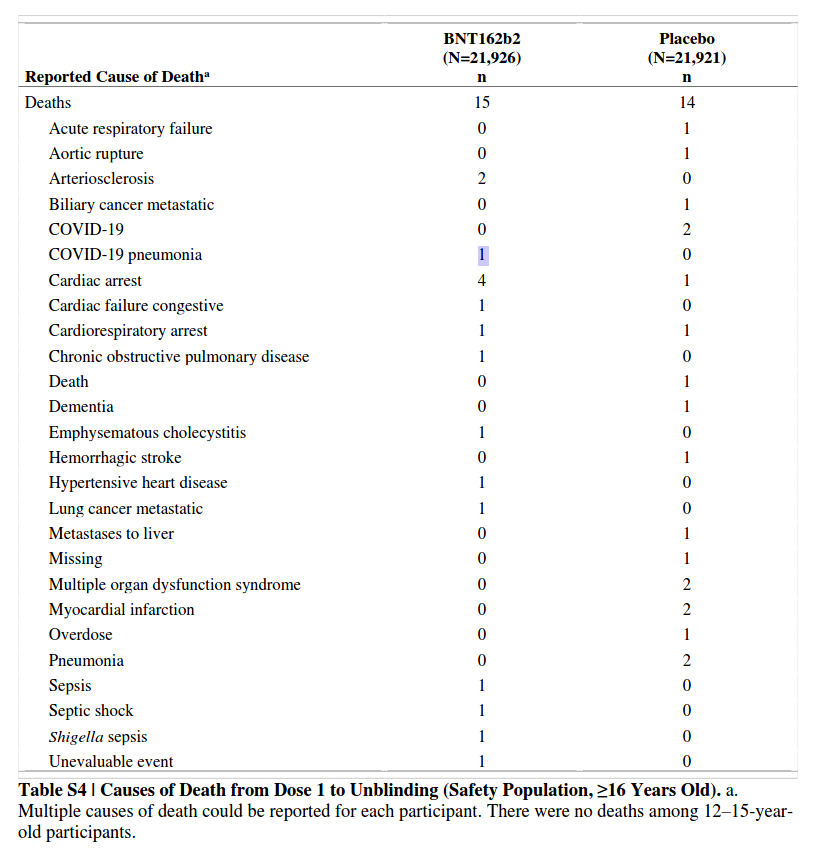
[Six Month Safety and Efficacy of the BNT162b2 mRNA SARS-2 Vaccine]
vaccine arm: 81 cases 1 death, 1.2% CFR
placebo arm: 873 cases 2 deaths 0.2% CFR
[]
[israeli study of over 104,000 patients showed that natural immunity is over 1,300% more effective and lower risk than the Pfizer biontech vaccine]
Comparing SARS-CoV-2 natural immunity to vaccine-induced immunity: reinfections versus breakthrough infections
>SARS-CoV-2-naïve vaccinees had a 13.06-fold (95% CI, 8.08 to 21.11) increased risk for breakthrough infection with the Delta variant compared to those previously infected, when the first event (infection or vaccination) occurred during January and February of 2021. The increased risk was significant (P<0.001) for symptomatic disease as well. When allowing the infection to occur at any time before vaccination (from March 2020 to February 2021), evidence of waning natural immunity was demonstrated, though SARS-CoV-2 naïve vaccinees had a 5.96-fold (95% CI, 4.85 to 7.33) increased risk for breakthrough infection and a 7.13-fold (95% CI, 5.51 to 9.21) increased risk for symptomatic disease. SARS-CoV-2-naïve vaccinees were also at a greater risk for COVID-19-related-hospitalizations compared to those that were previously infected.
https://www.medrxiv.org/content/10.1101/2021.08.24.21262415v1
Although the relative risk reduction (RRR) considers only participants who could benefit from the vaccine, the absolute risk reduction (ARR), which is the difference between attack rates with and without a vaccine, considers the whole population. ARRs tend to be ignored because they give a much less impressive effect size than RRRs: 1·3% for the AstraZeneca–Oxford, 1·2% for the Moderna–NIH, 1·2% for the J&J, 0·93% for the Gamaleya, and 0·84% for the Pfizer–BioNTech vaccines.
>ARR is also used to derive an estimate of vaccine effectiveness, which is the number needed to vaccinate (NNV) to prevent one more case of COVID-19 as 1/ARR. NNVs bring a different perspective: 76 for the Moderna–NIH, 78 for the AstraZeneca–Oxford, 80 for the Gamaleya, 84 for the J&J, and 117 for the Pfizer–BioNTech vaccines. The explanation lies in the combination of vaccine efficacy and different background risks of COVID-19 across studies: 0·9% for the Pfizer–BioNTech, 1% for the Gamaleya, 1·4% for the Moderna–NIH, 1·8% for the J&J, and 1·9% for the AstraZeneca–Oxford vaccines.
[]
>Effectiveness is the Israeli mass vaccination campaign using the Pfizer–BioNTech product. Although the design and methodology are radically different from the randomised trial,report an RRR of 94%, which is essentially the same as the RRR of the phase 3 trial (95%) but with an ARR of 0·46%, which translates into an NNV of 217 (when the ARR was 0·84% and the NNV was 119 in the phase 3 trial). This means in a real-life setting, 1·8 times more subjects might need to be vaccinated to prevent one more case of COVID-19 than predicted in the corresponding clinical trial
[]
>Uncoordinated phase 3 trials do not satisfy public health requirements; platform trials designed to address public health relevant questions with a common protocol will allow decisions to be made, informed by common criteria and uniform assessment.
>These considerations on efficacy and effectiveness are based on studies measuring prevention of mild to moderate COVID-19 infection; they were not designed to conclude on prevention of hospitalisation, severe disease, or death, or on prevention of infection and transmission potential.
https://www.thelancet.com/journals/lanmic/article/PIIS2666-5247(21)00069-0/fulltext
[]
BNT162b2 vaccine breakthrough: clinical characteristics of 152 fully-vaccinated hospitalized COVID-19 patients in Israel
> 152 patients were included, accounting for half of hospitalized fully-vaccinated patients in Israel. Poor outcome was noted in 38 patients and mortality rate reached 22% (34/152).
> Notable, the cohort was characterized by a high rate of comorbidities predisposing to severe COVID-19, including hypertension (108, 71%), diabetes (73, 48%), CHF (41, 27%), chronic kidney and lung diseases (37, 24% each), dementia (29, 19%), and cancer (36, 24%), and only 6 (%) had no comorbidities.
> Sixty (40%) of the patients were immunocompromised.
https://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(21)00367-0/fulltext
[]
Study shows post-jab cases more likely to be infected with virus strains that have emerged in recent months
>While everyone in the vaccinated group had a variant of concern, only 67 per cent of non-vaccinated individuals did. The study also showed that the vaccinated individuals infected with Covid had high viral loads.
>Dr Pavitra Roychoudhury, the lead author of the study, said the "prevailing understanding" was that while vaccine breakthrough cases would occur, they would be mild. "But in contrast to that, what we saw among our 20 samples was that a number of them actually had quite robust viral loads. That was concerning in the sense that there was definitely enough virus to sequence, and potentially there might be enough virus to transmit," she said.
https://archive.is/5bFTE
Pfizer's own statement over the efficacy of their product
>the final vaccine efficacy percentage may vary
https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-announce-vaccine-candidate-against
>if the vaccines are approved without an appropriate and accurate review of efficacy, then any potential acceptance or mandate of these vaccines is likely to be based on inaccurate evidence regarding the vaccine, namely that it will stop transmission of the virusfrom the vaccine recipient to others and/or that it will reduce COVID-19 disease and deaths. The Pfizer/BioNTech trial protocol and other trial protocols are currently not designed to determine whether either of those objectives can be met
https://archive.vn/15Eg6
>Although the present study was performed using a live VNT, it focused only on the humoral response and other experiments are needed to assess the overall immune process including T-cell immune response.
https://www.medrxiv.org/content/10.1101/2021.05.11.21256578v1
>A second limitation of the study is that no serological correlate of protection against COVID-19 has been defined.
>Therefore, predictions about vaccine efficacy based on neutralization titers require assumptions about the levels of neutralization and roles of humoral and cell-mediated immunity in vaccine-mediated protection.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7852264
As seen even in naturally infected people, seropositivity has no guarantees on actually protecting you with the latest circulating strains, and could possibly even work against you (moreso if you previously encountered a very outdated shape of the pathogen, like in the vaccines that lag behind the actual strains shapes by ~1.5 year (slightly less for the "updated" booster shots which take months to pass through trials before distribution anyway))
Incidence of SARS-2 recurrence among large cohort of healthcare employees: neither prior exposure to SARS-CoV-2, as indicated by positive IgG status, nor prior infection with SARS-2 appear to be protective and, instead, are evidenced to increase likelihood of SARS-2 recurrence
> Regressions adjusted for documented disparities showed no difference in COVID-19 infection by IgG status (OR=1.19; P = .3117) but significantly greater odds in COVID-19 recurrence among participants with a prior documented COVID-19 infection (OR=1.93; P < .0001).
> SARS-CoV-2 IgG antibodies and prior COVID-19 infection do not appear to offer meaningful protection against COVID-19 recurrence in healthcare workers.
> Participants with a prior documented COVID-19 infection had 2.47[2.09,2.92] times greater crude odds and 1.93[1.62,2.29] times greater adjusted odds of recurrence of COVID-19 (both at P < .0001).
> Younger age and more exposure to COVID-19 via clinical role category were significant predictors of COVID-19 recurrence in adjusted analyses, both significant at P < .0001
> After adjusting for demographic factors and clinical role, unlike positive IgG status, having a prior COVID-19 infection remained significant to the prediction of COVID-19 recurrence, reflecting nearly double the odds of recurrence.
[...]
> Findings reveal that neither prior exposure to SARS-CoV-2, as indicated by positive IgG status, nor prior infection with COVID-19 appear to be protective and, instead, are evidenced to increase likelihood of COVID-19 recurrence.
Two, large, credible (with possible conflict of interests favouring of the vaccine efficacy), studies have shown the vaccine to achieve less than 1% reduction in deaths between groups of vaccinated and unvaccinated.
>The New England Journal of Medicine
1,193,236 participants
9 out of 596,618 people died in the vaccinated group
32 out of 596,618 people died in the unvaccinated group
The press will claim “72% reduction is deaths!” (32 down to 9) ignoring the group size of 1.2M.
The truth is, that just 0.0039% more people in the unvaccinated group died, compared to the vaccinated group.
>The Lancet - Vaccine Results in Israel
6,538,911 total participants
138 out of 4,714,932 people died in the vaccinated group
715 out of 1,823,979 people died in the unvaccinated group
Do the math and you'll find that just 0.036% more deaths occurred in the unvaccinated group
https://www.nejm.org/doi/full/10.1056/NEJMoa2101765
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)00947-8/fulltext
[Breakthrough infections?]
As expected, incredibly prevalent
>2021 July data from 7 U.S. states show the Delta variant is fueling a rise in breakthrough infections. At least 1 in every 5 new COVID cases in 6 of these states have involved vaccinated people, with higher % of hospitalizations and deaths among breakthrough
https://www.medicinenet.com/script/main/art.asp?articlekey=262711
>Israel-based Haaretz News reported today a study by the Maccabi Health Maintenance Organization confirmed 37 people tested positive for COVID-19 after receiving a third 'booster' vaccination.
https://www.precisionvaccinations.com/2021/08/18/israels-3rd-vaccination-cant-stop-covid-19-breakthrough-re-infections/
[List of current vaccines?]
https://covid19.trackvaccines.org
https://ourworldindata.org/covid-vaccinations
https://vac-lshtm.shinyapps.io/ncov_vaccine_landscape/
https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html
https://milkeninstitute.org/covid-19-tracker
[Side-effects? Adverse events? Complications?]
Reminder that vaccines may initiate immune over-reaction to adjuvants, surfactans, and the vectors, that's not mentioning that the presence of those will also train your immunity for the response timing being unsuitable for a real infection.
Adenoviruses (as vectors) may bind to platelets via CARs or proteoglycans, causing clots on accumulation, which may cascade to DIC (random small clots thorough the body) and thrombocytopenia.
https://jvi.asm.org/content/81/9/4866
Also some of the vectors used in the vaccines seen to, on the contrary to official vaccine manufacturer statements, replicate, contributing to the damages.
Digital PCR to quantify ChAdOx1 nCoV-19 copies in blood and tissues
>In order to address practical utility of the assay we tested plasma samples from nine individuals obtained 24, 72 and 168 hours post vaccination. We isolated cell-free cfDNA from blood plasma for each time point. DPCRs were performed in duplex reactions using the RPP30 gene as reference to verify presence of cell-free DNA. At both 24 and 72 hours post vaccination, we detected vector copies in all nine plasma samples, which contained variable amounts of cfDNA (not shown). Interestingly, in six of the nine individuals vector copy numbers were higher 72 hours as compared to 24 hours post vaccination (Figure 3a).
https://www.biorxiv.org/content/10.1101/2021.05.28.446155v1
>Sputnik V vaccine Ad5 vector is evidently replication competent. The makers apparently neglected to delete E1, so getting this vaccine means being infected with live adenovirus 5.
https://www.ctvnews.ca/health/coronavirus/brazil-health-regulator-rejects-russia-s-sputnik-vaccine-1.5403539
Lipid nanoparticles used in mRNA vaccs are toxic, carcinogenic and the garbage from them once they reach a cell gets to accumulate in organs, notably liver. Surfactants used are carcinogenic and toxic, too. And due to the fast mRNA deterioration rates outside of a freezer, your cells could produce some weird shit from the "damaged" blueprints that even vaccine distributors don't know how it would end like (CTRL+F "pfizer leak" for more on mRNA vaccines).
>There are several distinct mechanisms that control the quality of mRNAs and proteins during translation at the ribosome. mRNA quality control systems, nonsense-mediated decay, non-stop decay, and no-go decay detect premature stop codons, the absence of a natural stop codon, and stalled ribosomes in translation, respectively, and degrade their mRNAs. Defective truncated polypeptide nascent chains generated from faulty mRNAs are degraded by ribosome-associated protein quality control pathways.
>If aberrant proteins escape the quality control, they may misfold, form insoluble aggregates or amyloids, and result in many human diseases
https://archive.is/Pifs2
And all the vaccines make your body face the spike protein in one form or another (the vaccines that make your body face even more types of proteins from SARS-CoV-2, of course, put your health at even greater risk), and the spike initiates a lot of processes and cascades that lead to blood clots.
One of the fun ways of this happening is body creating anti-PF4 antibodies in response to immune challenge from the spike protein.
This only happens in severe SARS-2 or with vaccines at play, where thanks to adjuvants, surfactants and/or vectors, the immunogenic effect ends up being strong enough for the recipients to develop the antibodies that bind to PF4, which may bind to NETs https://www.biorxiv.org/content/10.1101/630483v1.full.pdf that anti-PF4 antibodies exactly make (neutrophils overactivated by antibody receptors induce inflammation, break down the blood vessels around, this activates platelets that "repair" damaged by autoimmunity blood vessels but end up clotting them and causing thrombi, which in sum constitutes neutrophil extracellular trap (NET) formation).
Normally anti-PF4 antibodies are only associated with long term heparin users, small prevalence in general population. If they express anti-PF4 ab, they should stop taking heparin.
Additional funny thing is that heparin is one of the treatments for severe/hospital SARS-2.
Non-replication subunit vaccines, while not effective at all against SARS-2 like all other SARS-2 vaccines, are, by design (at least looking at Novavax), seem to be least harmful one (but still harmful).
1. Cells won't get to produce anything weird before their death.
2. Cells won't get to express the spikes on their surface since they would not get to produce them, so they not get to lead to them clustering up into abomination syncytia formations thanks to the spikes they produced and have expressed interacting with the nearby cells' receptors.
3. Novavax has only 1 type of protein from SARS-CoV-2 to present to the immune system (S protein), that would be a less broad autoimmune response chance, especially in comparison to whole virus vaccines, inactivated or not.
The particles the spike subunits are attached to are lipids
In relation to adjuvant, it's soap like
>The spike protein is formulated with a soap-like molecule called Polysorbate 80 (aka Tween 80). This consists of a hydrocarbon tail – essentially the tail group of oleic acid – attached to a sugar like molecule – sorbitan – to which are attached short chains of ethylene oxide. The whole thing is what’s known as a non-ionic surfactant. It’s like soap, in that it has a hydrophobic tail group and a hydrophilic head group. But unlike soap or comment synthetic detergents, the head group is, although water soluble, uncharged. The net result is that in water Polysorbates-80 self-assembles into nanoscale droplets – micelles – in which the hydrophobic tails are buried in the core and the hydrophilic head groups cover the surface, interacting with the surrounding water. The shape and size of the micelles is set by the length of the tail group and the area of the head group, so for these molecules the optimum shape is a sphere, probably a few tens of nanometers in diameter.
http://www.softmachines.org/wordpress/?p=2562
https://novavax.com/sites/default/files/2020-09/FINAL_Maynard_RSV_2018_Poster_Ernie_FINAL_%28ELM%29.pdf
Also, for the mRNA vaccines, as mRNA was reported to degrade quickly outside of designated freezer conditions, for when there are "damages" in the participating parties of the translation processes, tl;dr you will get:
1. weird shit produced instead of full intact spike proteins, ranging from harmless protein garbage to, if RNA got to change into a coding sequence for a different protein, misfolded proteins
2. the protein garbage products may lead to mitochondrial dysfunction
3. also, misfolded proteins may lead to amyloid diseases anyway
https://archive.is/b1XRD
The chances that the sequence of RNA being changed to become coding sequence of a prion after degradation is unknown, but not impossible.
There are suggestions that prion-like domains found in the S protein, be it that they may participate exclusively in the viral entry or not and participate in pathogenicity, too...
Identified Prion-Like Domains (PrDs) in the α1 helix of SARS-CoV-2 receptor-binding domain (RBD) and ACE2 region that interact with RBD have important functional roles in viral adhesion and entry
>Compared with other viruses, a striking difference was observed in the distribution of prion-like domains in the spike protein, since SARS-CoV-2 was the only coronavirus with a prion-like domain found in the receptor-binding domain of the S1 region of the spike protein.
>The presence and unique distribution of prion-like domains in the SARS-CoV-2 receptor-binding domains of the spike protein is particularly interesting, since although the SARS-CoV-2 and SARS-CoV S proteins share the same host cell receptor, ACE2, SARS-CoV-2 demonstrates a 10- to 20-fold higher affinity for ACE2.
https://archive.vn/ysLJX
...S protein interaction with cells cascading into something that would help prion disease to emerge is not completely out of the question, like via interacting with endogenous retroviruses within the human genome (although this is still extremely far-fetched, given how there is, 1.7+ of a year since virus release, a lack of evidence to suggest prion disease from S protein interactions is at least plausible).
Possible retroviral origin of prion disease
>While retroviral genes themselves may be responsible for neuronal death, a retrovirus may also cause mutations in cellular genes. Hence, the prion gene may be altered by a retrovirus in the same way as a cellular proto-oncogene is altered to produce an oncogene, either by transduction or by integration of the provirus in its vicinity. In both cases, the resulting abnormal prion protein, acting as a catalyst, may induce the formation of amyloid plaques. In addition, a wild type retrovirus may recombine to the vesicular stomatitis virus (VSV) to give rise to a pseudotyped retrovirus able to induce spongiosis. It is reported here that in scrapie, a blood monocytoid cell proliferates in vitro. If confirmed in other species, this raises the question of the potential link between prion disease and leukemia. Indeed neurovirulent strains of murine leukemia virus, a slow acting retrovirus, are known to induce spongiform encephalopathies. A preliminary attempt to purify reverse transcriptase by chromatography, using the classical protocol, failed because of the presence of a prion-like protein secreted by the blood mononuclear cells which stuck to the phosphocellulose column. Therefore, if a retrovirus is present in prion diseases, it would be evidenced only in animals developing the disease in the absence of prion protein.
https://pubmed.ncbi.nlm.nih.gov/10221168
(Which may cascade into a loop)
Prions May Activate Retroviruses In Infected Brain Cells
https://www.sciencedaily.com/releases/2007/09/070907095625.htm
Given that the whole prion disease form spike protein (specifically misfolding of PrP, not to be confused with amyloid formation or neurodegenerative complications in general) is based on nothing more than on opinion...
A Possible Link to Prion Diseases and Neurodegeneration
>Prion diseases are a collection of neurodegenerative diseases that are induced through the misfolding of important bodily proteins, which form toxic oligomers that eventually precipitate out as fibrils causing widespread damage to neurons. Stanley Prusiner first coined the name `prion’ to describe these misfolded proteins
>researchers have identified a signature motif linked to susceptibility to misfolding into toxic oligomers, called the glycine zipper motif. It is characterized by a pattern of two glycine residues spaced by three intervening amino acids, represented as GxxxG.
>The bovine prion linked to MADCOW has a spectacular sequence of ten GxxxGs in a row (see uniprot.org/uniprot/P10279).
>More generally, the GxxxG motif is a common feature of transmembrane proteins, and the glycines play an essential role in cross-linking α-helices in the protein
>Prion proteins become toxic when the α-helices misfold as β-sheets, and the protein is then impaired in its ability to enter the membrane
>Glycines within the glycine zipper transmembrane motifs in the amyloid-β precursor protein (APP) play a central role in the misfolding of amyloid-β linked to Alzheimer’s disease
>APP contains a total of four GxxxG motifs.
>When considering that the SARS-CoV-2 spike protein is a transmembrane protein, and that it contains five GxxxG motifs in its sequence (see uniprot.org/uniprot/P0DTC2), it becomes extremely plausible that it could behave as a prion
https://ijvtpr.com/index.php/IJVTPR/article/view/23/51
>extremely plausible
(pf, the five GxxxG motifs don't even seem to go in a row)
...what actually likely, is vaccines or the virus itself accelerating already existing prion diseases (as well as inducing neurodegenerative diseases, with accelerating them as well).
Creutzfeldt-Jakob disease in a man with SARS-2
>Depending on local milieu, astrocytes may be induced to assume one of two distinct reactive forms: a neurotoxic A1 phenotype and a neuroprotective A2 phenotype.
>A1 astrocytes potentiate death of neighboring neurons and oligodendrogliocytes
>Il-1, TNF and C1q are collectively necessary and sufficient for A1 astrocyte activation
>In prion disease, A1 reactive astrocytes are thought to be neurotoxic by mediating neuronal damage of adjacent neuronal processes and serving as foci for PrPSc propagation
>Pre-clinical CJD is marked by retention of region-specific homeostatic identities of glia, including astrocytes. During the transition to clinical CJD these region-specific signatures are lost and replaced by a neuroinflammatory transcriptome signature that affects astrocyte sub-populations in a region-dependent manner
https://archive.is/kmjIy
Just a side note, prions are something that's quite hard to aerosolize, they'd have to travel through the cardiac vessels, into the capillaries, then diffuse through the capillaries into the alveoli, then they'd have to condense enough to form granulomas.
Prions spreading via urine, feces or blood is much more likely.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6264140
Towards Understanding ChAdOx1 nCov-19 Vaccine-induced Immune Thrombotic Thrombocytopenia (VITT)
> ChAdOx1 nCoV-19 vaccine constituents (i) form antigenic complexes with PF4, (ii) EDTA increases microvascular permeability, and (iii) vaccine components cause acute inflammatory reactions.
> Antigen formation in a proinflammatory milieu offers an explanation for anti-PF4 antibody production. High-titer anti-PF4 antibodies activate platelets and induce neutrophil activation and NETs formation, fueling the VITT prothrombotic response.
https://www.researchsquare.com/article/rs-440461/v1
Inactivation of SARS-CoV-2 by β-propiolactone Causes Aggregation of Viral Particles and Loss of Antigenic Potential
https://www.biorxiv.org/content/10.1101/2021.04.22.441045v1
[]
β-propiolactone is carcinogenic, if there are vaccine production byproducts in the frozen vials of the vaccine where the virus was inactivated with it, it could get into your body on injection and stay for a while before its half-life expires
https://pubs.acs.org/doi/10.1021/acs.chemrestox.9b00389
[]
That includes inactivated vaccines from china
>Beta-propiolactone inactivated SARS-CoV-2 vaccines have been mainly developed in China
https://www.frontiersin.org/articles/10.3389/fimmu.2020.602256/full
And so,
[Some of the reported adverse events (including the ones where, supposedly, placebo or anything else were "reported" to be the cause)]
(may include overlaps of vaccine pathology with virus pathology in vaccinated who were infected)
[Studies on adverse events]
SARS-CoV-2 mRNA Vaccination-Associated Myocarditis in Children Ages 12-17: A Stratified National Database Analysis
>Post-vaccination CAE rate was highest in young boys aged 12-15 following dose two. For boys 12-17 without medical comorbidities, the likelihood of post vaccination dose two CAE is 162.2 and 94.0/million respectively. This incidence exceeds their expected 120-day COVID-19 hospitalization rate at both moderate (August 21, 2021 rates) and high COVID-19 hospitalization incidence.
>A total of 257 CAEs were identified. Rates per million following dose 2 among males were 162.2 (ages 12-15) and 94.0 (ages 16-17); among females, rates were 13.0 and 13.4 per million, respectively. For boys 12-15 without medical comorbidities receiving their second mRNA vaccination dose, the rate of CAE is 3.7-6.1 times higher than their 120-day COVID-19 hospitalization risk as of August 21, 2021 (7-day hospitalizations 1.5/100k population) and 2.6-4.3-fold higher at times of high weekly hospitalization risk (2.1/100k), such as during January 2021. For boys 16-17 without medical comorbidities, the rate of CAE is currently 2.1-3.5 times higher than their 120-day COVID-19 hospitalization risk, and 1.5-2.5 times higher at times of high weekly COVID-19 hospitalization
https://www.medrxiv.org/content/10.1101/2021.08.30.21262866v1
Increased relative risk of myocarditis in adolescents (22x) and 18-29 years old (7x) vs older people
>Noteworthy, Israel’s spontaneous reports are not transmitted to VigiBase.
>Following the widespread adoption of community-based mitigation measures to reduce the transmission of SARS-CoV-2, the percentage of other respiratory diseases, such as influenza infection, has declined to historically low levels.
>Therefore, we can hypothesize that the background risk for myocarditis is probably lower than usual.
>Potential mechanisms for these observed reactions are currently unknown, but several hypotheses may be considered.
>The first is that incidence appears to be higher in situations related to higher immune reactivity.
>This may be related to greater adaptive immune response in younger individuals, which in turn may lead to greater increases of CD4+ Th17+ cell populations, which predisposes individuals to developing myocarditis.
>Second is the possibility that mRNA in these vaccines may enhance autoimmunity.
>mRNA is known to be a self-adjuvant for innate immune responses, and this may help to explain their immunogenicity, and may also trigger excessive immune responses in some individuals, especially when there may be presence of a cross-reacting antigen.
https://www.medrxiv.org/content/10.1101/2021.08.12.21261955v1
Association of Vaccine Type and Prior SARS-CoV-2 Infection With Symptoms and Antibody Measurements Following Vaccination Among Health Care Workers
>Prior SARS-CoV-2 exposure was associated with increased odds of clinically significant symptoms following dose 1 (OR, 4.38; 95% CI, 2.25-8.55) but not dose 2 (OR, 0.60; 95% CI, 0.36-0.99), after controlling for vaccine type, age, and sex.
https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2782821
A shot of SARS-2 vaccine may cause relapse of herpes zoster in some recipients
>Researchers in Israel found six cases of shingles in patients who received a SARS-2 vaccine shot
>Herpes zoster is caused by virus that lies inactive in the human body after a person has chickenpox
https://academic.oup.com/rheumatology/advance-article/doi/10.1093/rheumatology/keab345/6225015
SARS-2 Vaccination-Associated Myocarditis in Adolescents
>63 patients (mean age: 15.6 years!). 92% were male.
>All had received #COVIDVaccines &, except for one, presented following the 2nd dose.
>- 4 had dysrhythmia
>- 14% had mild left ventricular dysfunction
>- 88% had myocarditis
https://pediatrics.aappublications.org/content/early/2021/08/12/peds.2021-053427.long
Israel
"95% of the severe patients are vaccinated".
"85-90% of the hospitalizations are in Fully vaccinated people."
"We are opening more and more COVID wards."
"The effectiveness of the vaccine is waning/fading out"
https://twitter.com/RanIsraeli/status/1423322271503028228
[Bonus data]
Concerning results, Israel 2021 (Prof. Retsef Levi)
>MDA Emergency calls:
>25% increase in Cardiac arrests & Heart attacks (16-29).
>83.6% increase in Heart attacks (Women 20-29).
>According to the study, this increase was correlated with Mass vaccination.
>Deaths in Israel 2020 (0-19)->21% lower compared to previous years.
>Deaths in Israel 2021 (0-19)->low at the peak of the pandemic (Jan-Feb).
>Since then?
>~35% (10-19) got vaccinated,
>+we had more deaths (0-19)&Cardiac arrests.
>At least 30% of them were exposed before vaccination!
https://web.archive.org/web/20210829140506/https://twitter.com/RanIsraeli/status/1425002893116166144
https://drive.google.com/file/d/1QT2uUC4j9I2cVpsD1prkScBg0gUqI52x/view
[Vaccine adverse event trackers]
Cases where Vaccine targets SARS-2 (COVID19) and Patient Died
https://www.medalerts.org/vaersdb/findfield.php?EVENTS=on&VAX=(COVID19)&VAXTYPES=(COVID-19)&DIED=Yes
Handy access to VAERS
Tracker for reports from EU (Eudra Vigilance)
http://www.adrreports.eu
Tracker for reports from Australia (TGA)
https://apps.tga.gov.au/Prod/daen/daen-report.aspx
Tracker for reports from UK (Yellow Card)
https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions
A nice, long and diverse list on complications from pfizer, from UK
Moderna vaccine
https://dap.ema.europa.eu/analytics/saw.dll?PortalPages&PortalPath=%2Fshared%2FPHV%20DAP%2F_portal%2FDAP&Action=Navigate&P0=1&P1=eq&P2=%22Line%20Listing%20Objects%22.%22Substance%20High%20Level%20Code%22&P3=1+40983312
Pfizer biontech vaccine
https://dap.ema.europa.eu/analytics/saw.dll?PortalPages&PortalPath=%2Fshared%2FPHV%20DAP%2F_portal%2FDAP&Action=Navigate&P0=1&P1=eq&P2=%22Line%20Listing%20Objects%22.%22Substance%20High%20Level%20Code%22&P3=1+42325700
Astra Zeneca
https://dap.ema.europa.eu/analytics/saw.dll?PortalPages&PortalPath=%2Fshared%2FPHV%20DAP%2F_portal%2FDAP&Action=Navigate&P0=1&P1=eq&P2=%22Line%20Listing%20Objects%22.%22Substance%20High%20Level%20Code%22&P3=1+40995439
Jansen
https://dap.ema.europa.eu/analytics/saw.dll?PortalPages&PortalPath=%2Fshared%2FPHV%20DAP%2F_portal%2FDAP&Action=Navigate&P0=1&P1=eq&P2=%22Line%20Listing%20Objects%22.%22Substance%20High%20Level%20Code%22&P3=1+42287887
["Underreporting" is one of the main limitations of VAERS and other similar voluntary adverse event reporting systems]
>fewer than 1% of vaccine adverse events are reported. Low reporting rates preclude or slow the identification of “problem” drugs and vaccines that endanger public health.
https://censored.vaxcalc.org/wp-content/uploads/2019/06/r18hs017045-lazarus-final-report-2011.pdf
[]
>Guide to Interpreting VAERS Data
>VAERS Data Limitations
>"Underreporting" is one of the main limitations of passive surveillance systems, including VAERS. The term, underreporting refers to the fact that VAERS receives reports for only a small fraction of actual adverse events. The degree of underreporting varies widely.
https://vaers.hhs.gov/data/dataguide.html
https://vaxopedia.org/2017/08/26/underreporting-of-side-effects-to-vaers/
https://cran.r-project.org/web/packages/vaersNDvax/README.html
https://vaccinesafetycouncilminnesota.org/vaccine-injuries/
Also, there are anecdotal reports on medical workers not filling the VAERS reports
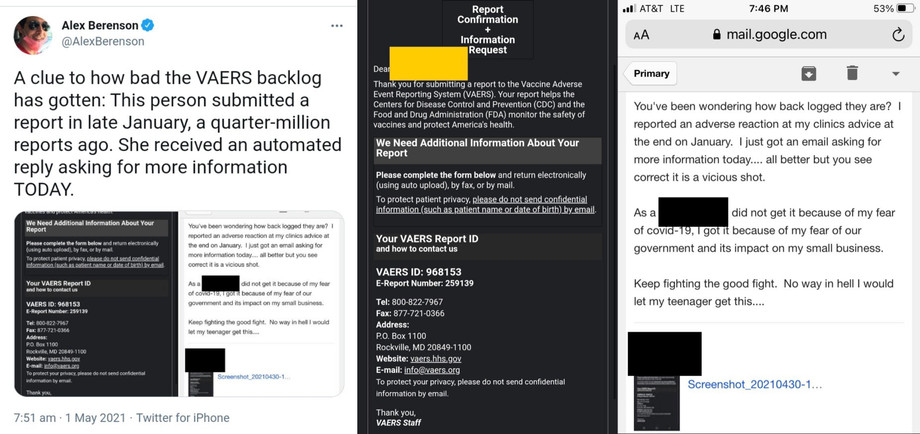
Along with more credible reports on VAERS still being overloaded and backlogged beyond any reason
Along with VAERS deleting at least tens of thousands of adverse event reports (suggesting either quality control of the data, or censorship (maybe both))
https://twitter.com/NormaSco19/status/1404899460942008324
https://odysee.com/@AnimatedSkeptic:2/150k-records-deleted-from-VAERS-covid-database
[Vaccine deaths dumps]
https://archive.4plebs.org/pol/thread/311387886
https://archive.4plebs.org/pol/thread/318153250/#318154390
[Miscarriage reports]
https://archive.4plebs.org/pol/search/text/vaccine%2A%20miscarriage%2A%20vaers/
https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-12/slides-12-19/05-COVID-CLARK.pdf
Very huge page with all the latest available reports on effects from all the SARS-CoV-2 vaccines
https://wonder.cdc.gov/controller/datarequest/D8;jsessionid=ADA8043B580F4511B0B1DFFD43CD?stage=results&action=sort&direction=MEASURE_DESCEND&measure=D8.M6
[]
[slightly outdated] A Long List of Recorded Adverse Reactions to the Experimental SARS-CoV-2 Vaccine in .pdf
https://femto.pw/u26k
[]
Original official source where you can make your own query
https://wonder.cdc.gov/vaers.html

Source:
fda.gov/media/143557/download.jpg
A 3.5-fold increase in the risk of myocarditis by 3.5 in the 21 days after the 1st dose 10-fold (!) within 7 days of 2nd dose
Updated CDC data - June 23 2021
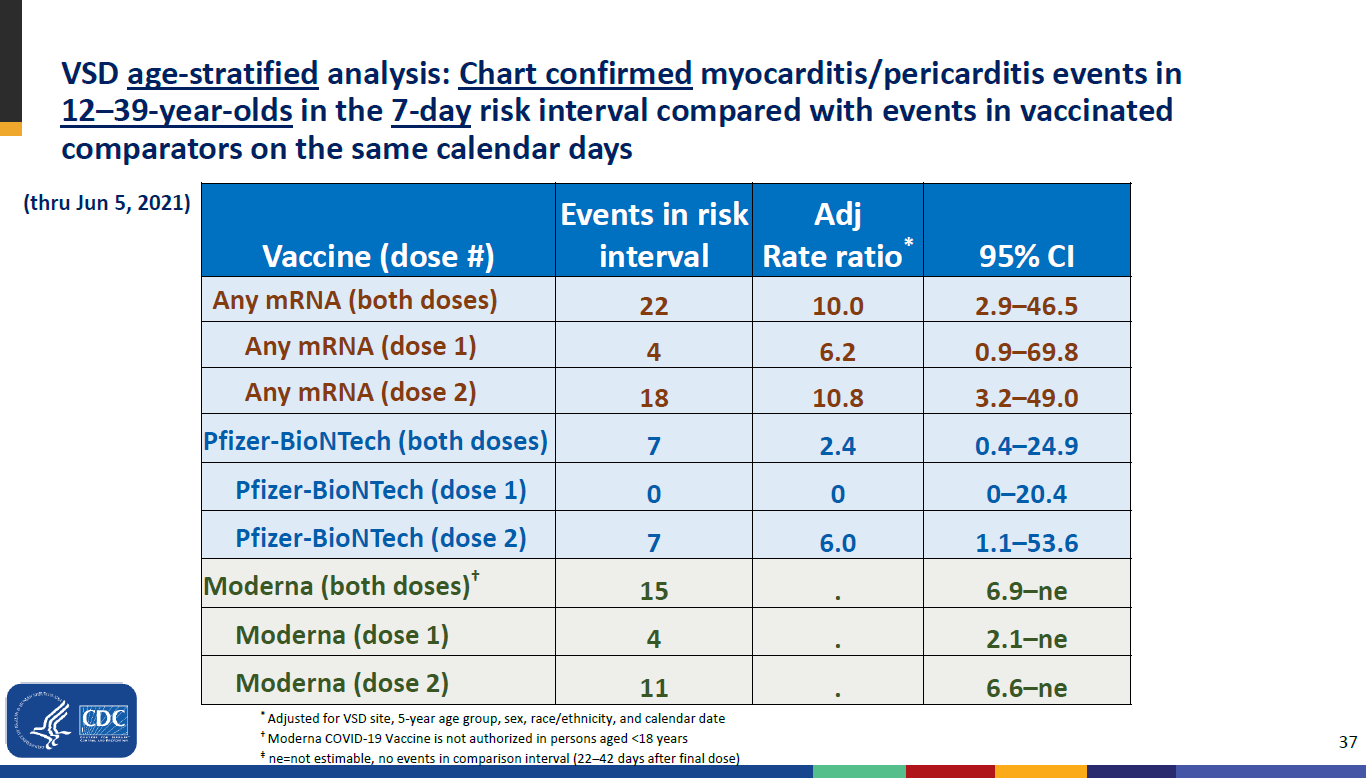
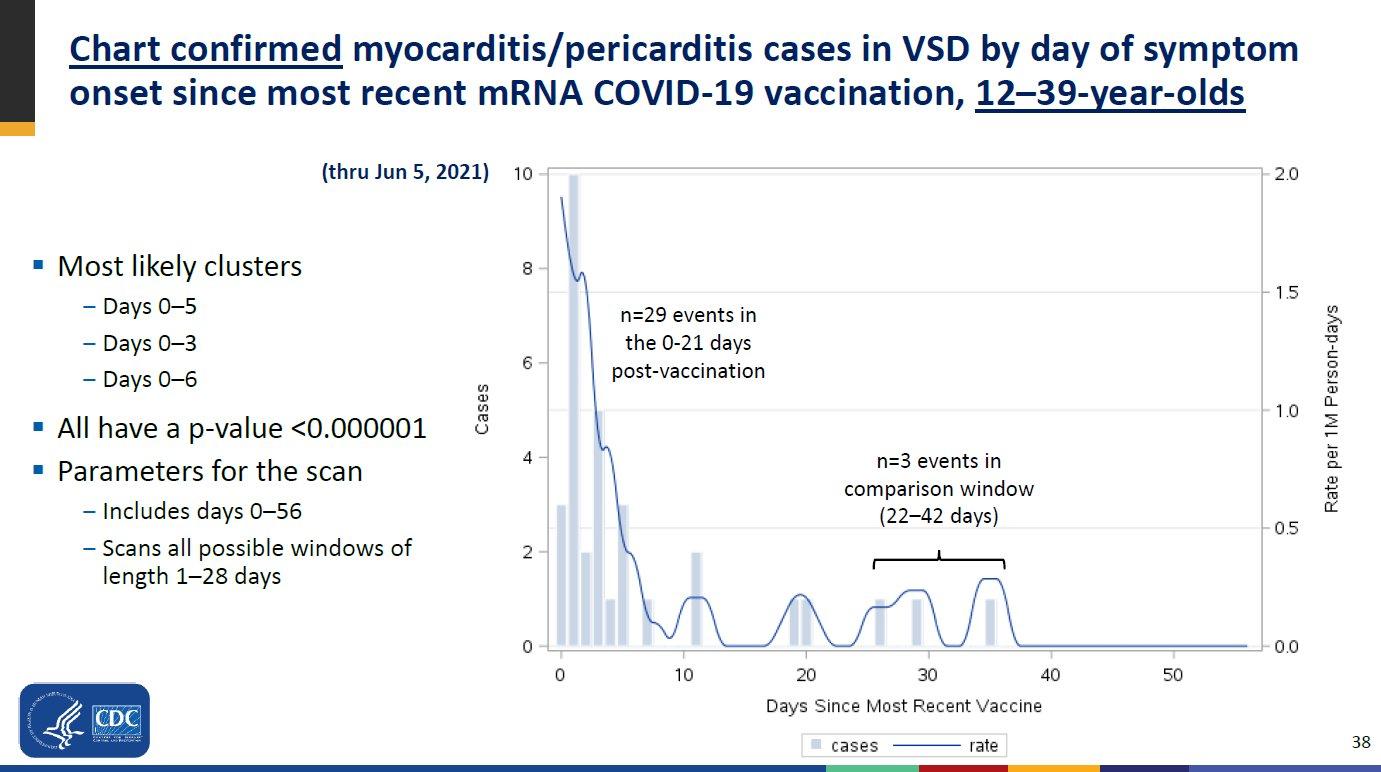
https://web.archive.org/web/20210714092747/https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/03-COVID-Shimabukuro-508.pdf
Death, Disability Among Side Effects of Chinese SARS-2 Vaccines, Leaked Documents Reveal
https://www.biospace.com/article/death-disability-among-side-effects-of-chinese-covid-19-vaccines-leaked-documents-reveal
https://archive.is/l4acg
https://archive.is/gVMEg
Russia SARS-CoV-2 vaccine adverse events up to 30th June 2021
https://justpaste.it/1yvtx
Norway warns frail patients over 80 of vaccine risks after vaccine-related deaths
https://archive.vn/XkeoF
Israel has been hailed for its speedy and efficient mass inoculation program, which has vaccinated a staggering 20 percent of the country’s population since the drive began at the end of December. For a handful of Israelis, however, the initiative has led to some unexpected health scares. At least 13 people have reported mild facial paralysis after receiving the Pfizer/BioNTech jab, Israeli outlet Ynet reported, citing the Health Ministry, adding that officials believe the number of such cases could be higher.
https://archive.today/qt3yI
51 adverse reactions reported & 1 person hospitalized in Delhi as India begins world’s largest Covid-19 vaccination program
https://archive.vn/iQj4a
Peru says china's Sinopharm may resume coronavirus vaccine trial after volunteer's illness
>The Peruvian health minister said on Wednesday that China’s Sinopharm could resume a trial for its coronavirus vaccine in the hard-hit Andean nation, just days after authorities suspended the tests to better understand why a volunteer had fallen ill.
>Health authorities announced over the weekend that the Sinopharm trial would be temporarily halted as a safety measure after a volunteer experienced decreased strength in his legs, among other symptoms.
>“We have had several meetings with Sinopharm and ... the suspension has been lifted today (Wednesday),” Health Minister Pilar Mazzetti said.
>Sinopharm Group Co Ltd, which is conducting its trials in Peru with some 12,000 volunteers, was about to complete the first stage of the trials in the next few days, and had plans to administer a second dose of its vaccine in the coming weeks.
>Peru’s government said on Tuesday negotiations with Sinopharm to purchase COVID-19 vaccines are “well advanced.”
https://archive.vn/ettBl
Four team members at Advocate Condell Medical Center in Libertyville experienced reactions shortly after receiving the Pfizer SARS-CoV-2 vaccination. Their symptoms included tingling and elevated heartrates, the hospital said in a statement.
https://archive.vn/YUMGB
[USA] Fairbanks clinician is third Alaskan with adverse reaction to SARS-CoV-2 vaccine
https://archive.vn/lE5Bt
2 Alaska Health Workers Got Emergency Treatment After Receiving Pfizer’s Vaccine
https://archive.vn/ovlo6
Four trial volunteers who got Pfizer's SARS-CoV-2 vaccine developed Bell's palsy - but FDA denies that the temporary facial paralysis was caused by the shot
https://archive.vn/COsU0
Priest Dies After Participating In Moderna COVID Vaccine Trial
https://www.zerohedge.com/geopolitical/philadelphia-priest-dies-after-participating-moderna-covid-vaccine-trial
AstraZeneca SARS-2 vaccine trial volunteer has died
https://archive.vn/UyfKD
[OCT 13 2020] Johnson & Johnson’s coronavirus vaccine trial is paused after ‘adverse event’ in a participant
>“We must respect this participant’s privacy,” the company said in a statement late Monday. “We’re also learning more about this participant’s illness, and it’s important to have all the facts before we share additional information.”
https://archive.vn/dk2ew
>Moderna
Mild fever after taking the first shot of the mRNA-1273 vaccine. "Full-on COVID-like symptoms".
There were other participants who also experienced similar side-effects
>Pfizer
Mild side effects after getting the first shot of the vaccine, while some reported side-effects after taking the second jab
>AstraZeneca [BTW they accidentally made the dosaging smaller]
At least 2 participants experienced transverse myelitis, which is an inflammatory syndrome that affects the spinal cord.
>Johnson & Johnson
Unexplained illness injection.
https://archive.vn/ZaDka
Side effects of the Moderna and Pfizer SARS-CoV-2 vaccines
>Neither company reported side effects that affected less than 2% of participants in their press releases but more detailed data will be released.
>Pfizer reported 3.8% of the recipients felt fatigue and 2% experienced headache, based on preliminary data from its Phase 3 trial. These symptoms are classified as Grade 3 or “severe” adverse events because they can interfere with daily activity.
>Moderna reported more Grade 3 side effects. There was fatigue in 9.7% of recipients, muscle pain in 8.9%, joint pain in 5.2%, headache in 4.5%, pain in 4.1%, and redness at the injection site in 2%.
https://archive.vn/kBTQh
[Efficacy of vaccines by type?]
Current subunit/vector/mRNA vaccines present only the spike protein to the immune system of the recipient, which would not be enough to prime the immunity for clearance properly:
>9 identified T cell epitopes were derived from the ORF1ab (n=4), S protein (n=3), membrane protein (n=1), and nucleoprotein (n=1). Strikingly, the CD8 T cell responses specific for the epitopes derived from ORF1ab were of significantly higher magnitude compared to the CD8 T cell responses towards the spike, membrane and nucleoprotein combined, was not due to increased relative expression of ORF1ab compared to other proteins
https://www.researchsquare.com/article/rs-33197/v2
But inactivated vaccines offer higher risks of autoimmune problems due to more proteins being present that could trigger pathogenic priming, and live attenuated virus vaccines also can replicate, act with the most virulence and possibly mutate back to more virulent/pathogenic form (may as well just get infected with the real virus, but only after souping up on prohyplaxis).
[Pathogen priming / autoimmune complications]
Note that vaccination campaigns push animal safety studies so much because there is less homology between animal proteins and SARS-2 virus proteins than there is between human proteins and SARS-2 virus proteins, and so the effects and damage from pathogenic priming (where the immune system ends up attacking human proteins which are similar to the virus proteins) within animals would be comparably minimal, and suitable for advertising the vaccines.
Pathogenic priming likely contributes to serious and critical illness and mortality in COVID-19 via autoimmunity
>Homology (similarity) between human and viral proteins is an established factor in viral- or vaccine-induced autoimmunity.
>Immunogenic peptides in viruses or bacteria that match human proteins are good candidates for pathogenic priming peptides
>If all of the parts of the epitopes that are homologous to human proteins are excluded from consideration due to risk of pathogenic priming, the remaining immunogenic parts of the epitopes may be still immunogenic and remain as potentially viable candidates for vaccine development
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7142689/
Epitope mimicry analysis of SARS-COV-2 surface glycoproteins and envelope proteins and human lung proteins
https://pubmed.ncbi.nlm.nih.gov/33588349
IgA autoantibodies target pulmonary surfactant in patients with severe COVID
>we established a prospective observational cohort to study lung specific autoantibodies (auto-Abs). Incubation of plasma from severe COVID-19 patients with healthy human lung tissue revealed the presence of IgA antibodies binding to surfactant-producing pneumocytes. Enzyme-linked immunosorbent assays (ELISA) and protein pull-downs using porcine surfactant confirmed the presence of auto-Abs binding to surfactant proteins in severe COVID-19 patients. Mass spectrometry and ELISAs with recombinant proteins identified IgA auto-Abs that target human surfactant proteins B and C. In line with these findings, lungs of deceased COVID-19 patients showed reduced pulmonary surfactant
https://www.medrxiv.org/content/10.1101/2021.02.02.21250940v1
[Molecular Mimicry] between SARS-CoV-2 spike glycoprotein and mammalian proteomes: implications for the vaccine
>Of the 33 peptides from SARS-CoV-2 that mimic the human reference proteome, 20 peptides are not found in any previous human-infecting coronavirus (SARS, MERS, or seasonal HCoVs)
>Such a peptide commonality is unexpected and highly improbable from a mathematical point of view
https://archive.vn/PG6b6
[Human–viral molecular mimicry] Benchmarking evolutionary tinkering underlying human–viral molecular mimicry shows multiple host pulmonary–arterial peptides mimicked by SARS-CoV-2
https://archive.vn/Lwr9k
Coronavirus associated molecular mimicry common to SARS-CoV-2 peptide
https://www.biorxiv.org/content/10.1101/2021.01.28.428642v1
New-Onset IgG Autoantibodies in Hospitalized Patients with COVID
https://www.medrxiv.org/content/10.1101/2021.01.27.21250559v1
Distinct Autoimmune Antibody Signatures Between Hospitalized Acute COVID Patients, SARS-CoV-2 Convalescent Individuals, and Unexposed Pre-Pandemic Controls
https://www.medrxiv.org/content/10.1101/2021.01.21.21249176v1
An Autoantigen Atlas from Human Lung HFL1 Cells Offers Clues to Neurological and Diverse Autoimmune Manifestations of SARS-2
https://www.biorxiv.org/content/10.1101/2021.01.24.427965v1
SARS-CoV2 infection as a trigger of humoral response against apolipoprotein A-1
>Anti-Spike domain 1 (SD1) IgGs, anti-apoA-1 IgGs and against mimic peptides, as well as cytokines were assessed by immunoassays on a case-control (n=101), an intensive care unit (ICU; n=126) with a 28-days follow-up, and a general population cohort (n=663) with available samples in the pre and post-pandemic period.
>Linear sequence homologies and antibodies cross-reactivity between apoA-1, TLR2, and Spike epitopes were identified.
https://www.medrxiv.org/content/10.1101/2021.02.12.21251298v1
[]
https://en.wikipedia.org/wiki/Apolipoprotein_A1#Clinical_significance
[]
>Apolipoprotein serum levels related to metabolic syndrome in patients with schizophrenia
https://archive.is/SohRh
[]
>Serum apolipoprotein A1: a predictor and prognostic biomarker in acute ischemic stroke
https://archive.is/GChFi
Neutralizing IFNL3 Autoantibodies in Severe SARS-2 Identified Using Molecular Indexing of Proteins by Self-Assembly
>we present Molecular Indexing of Proteins by Self Assembly (MIPSA), a technique that produces ORFeome-scale libraries of proteins covalently coupled to uniquely identifying DNA barcodes for analysis by sequencing.
>We used MIPSA to profile circulating autoantibodies from 55 patients with severe COVID-19 against 11,076 DNA-barcoded proteins of the human ORFeome library.
>MIPSA identified previously known autoreactivities, and also detected undescribed neutralizing interferon lambda 3 (IFN-λ3) autoantibodies.
>At-risk individuals with anti-IFN-λ3 antibodies may benefit from interferon supplementation therapies, such as those currently undergoing clinical evaluation
https://www.biorxiv.org/content/10.1101/2021.03.02.432977v1
[Will I still be spreading the virus after being inoculated?]
Yes.
Case cluster tracker from Singapore:
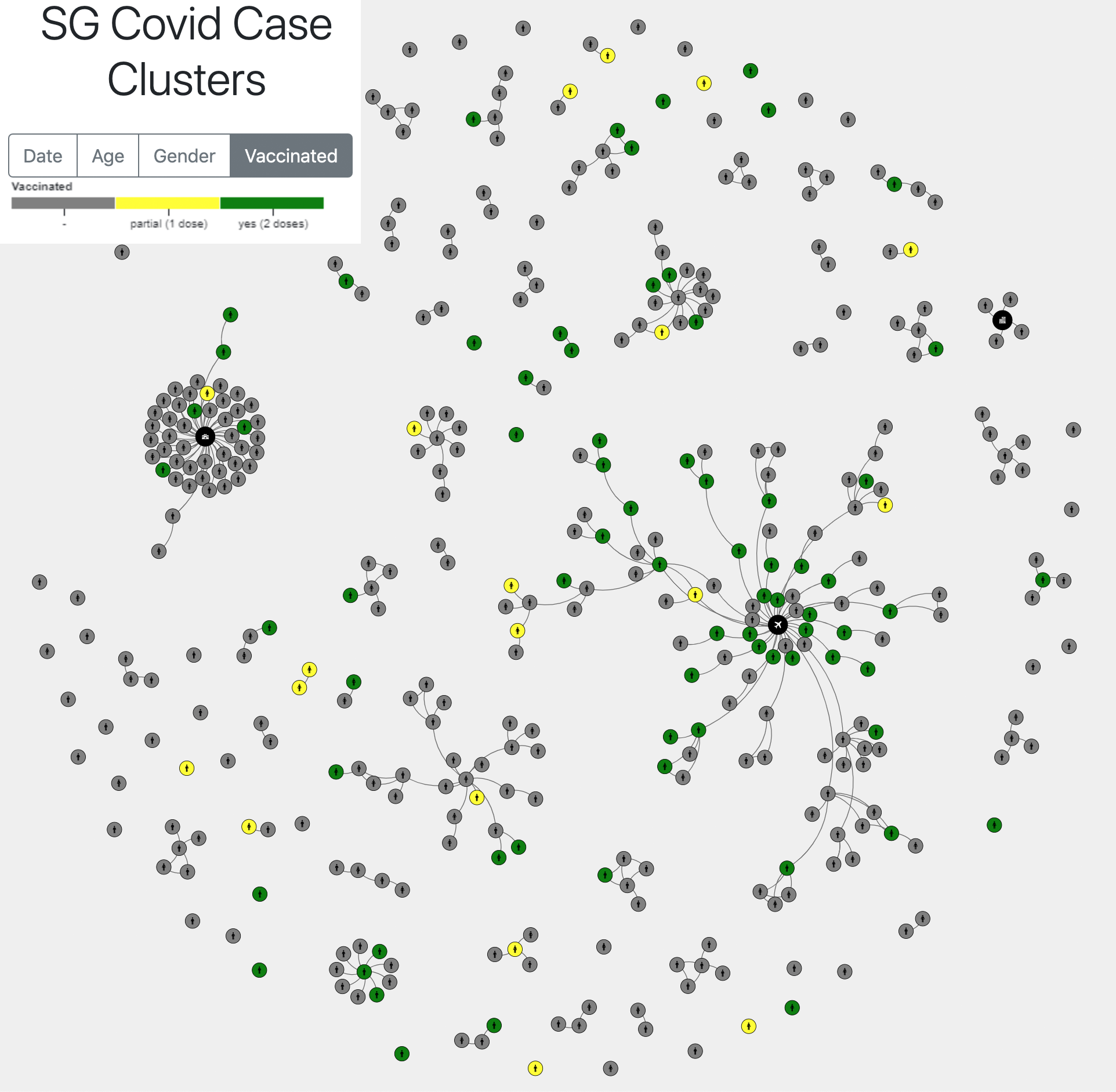
Moderna chief medical officer: Vaccinated adults could still infect the unvaccinated
https://archive.vn/tQFFk
UK: Coronavirus vaccine won't free you from self-isolation, says Government
>The jabs provide Covid-19 immunity but scientists are yet to prove this prevents recipients from carrying and spreading the virus.
https://archive.is/pxC2o
[For how long does the immunity from vaccines last?]
~6 months for humoral/antibody immunity, like from being infected naturally (but, effectively, the supposed immunity may not even last for a month, much less a week, all depends on how different. compared to what vaccine makes you face, will be the serotype of the strain/proteins you will run into).
>The Pfizer vaccine is thought to offer up to six months of immunity to Covid-19
https://archive.vn/AzoxQ
>Images have now been shared of a card patients will receive to prove they have received the jab - which has proved to be effective in 95 per cent of cases and offers up to six months of immunity
https://archive.vn/AzoxQ
Vaccines (or being naturally infected) should still provide cellular immunity (lower limit for how long exactly - unknown, presumably at least around up to 6 years for B cells, 17 years for T cells, as in SARS-CoV-1 survivors cellular immunity lasted for this long)
>patients who recovered from SARS have T cells that are specific to epitopes within different SARS-CoV proteins that persist for 11 years after infection11. Here, we collected PBMCs 17 years after SARS-CoV infection and tested whether they still contained cells that were reactive against SARS-CoV and whether these had cross-reactive potential against SARS-CoV-2 peptides. PBMCs from individuals who had resolved a SARS-CoV infection (n = 15) were stimulated directly ex vivo with peptide pools that covered the N protein of SARS-CoV (N-1 and N-2), NSP7 and NSP13 (Fig. 3a). This revealed that 17 years after infection, IFNγ responses to SARS-CoV peptides were still present and were almost exclusively focused on the N protein rather than the NSP peptide pools
https://archive.vn/tXcUQ
Although in effectiveness of cellular immunity against T-cell resistant mutations of SARS-CoV-2 is unknown
>D614G strain with a I472V mutation on it that is also fast growing in the US and Europe. Not only antibody resistant but also more infectious, and there are are now studies underway that might indicate that it's also T-cell resistant
thailandmedical.news/news/covid-19-latest-more-antibody-resistant-sars-cov-2-mutated-strains-emerging-and-increasing-in-circulation
[Is vaccines' efficacy affected by virus mutating?]
Why, yes.
>As reinfection occurs with other strains, this is especially bad news for vaccine development as it means there won't be any protection from reinfection provided for other strains by the antibodies created on the original virus vaccine
https://archive.vn/V89m4
The Potential for SARS-CoV-2 to Evade Both Natural and Vaccine-induced Immunity
https://www.biorxiv.org/content/10.1101/2020.12.13.422567v1
>COVID-19 virulence may be more severe in Europe and North America due to coinfection with different SARS-CoV-2 strains leading to genomic recombination which might be challenging for current treatment regimens and vaccine development
https://archive.vn/vBTo3
SARS-CoV-2 will evolve quickly to evade widely deployed spike RBD-targeting monoclonal antibodies, requiring combinations with at least three antibodies to suppress viral immune evasion
https://archive.vn/X4tL8
>D614G strain with a I472V mutation on it that is also fast growing in the US and Europe. Not only antibody resistant but also more infectious, and there are are now studies underway that might indicate that it's also T-cell resistant
thailandmedical.news/news/covid-19-latest-more-antibody-resistant-sars-cov-2-mutated-strains-emerging-and-increasing-in-circulation
[Specialists' concerns about this] Monitor for COVID-19 vaccine resistance evolution during clinical trials
https://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.3001000
[Vaccine shedding? S protein shedding? Shedding of vaccine-resistant strains upon infection?]
All seem to occur to some extent, even Pfizer notes "environmental exposure" as something as to what purposefully needs to be ignored to gain "good" data.
>Exposure to the study intervention under study during pregnancy or breastfeeding and occupational exposure are reportable to Pfizer Safety within 24 hours of investigator awareness.
>An EDP occurs if: A female is found to be pregnant while being exposed or having been exposed to study intervention due to environmental exposure. Below are examples of environmental exposure during pregnancy:
>A female family member or healthcare provider reports that she is pregnant after having been exposed to the study intervention by inhalation or skin contact.
>A male family member or healthcare provider who has been exposed to the study intervention by inhalation or skin contact then exposes his female partner prior to or around the time of conception.
>If EDP occurs in the setting of environmental exposure, the investigator must report information to Pfizer Safety using the Vaccine SAE Report Form and EDP Supplemental Form. Since the exposure information does not pertain to the participant enrolled in the study, the information is not recorded on a CRF.
>When exposure during breastfeeding occurs in the setting of environmental exposure, the exposure information does not pertain to the participant enrolled in the study, so the information is not recorded on a CRF.
>Occupational Exposure
>An occupational exposure occurs when a person receives unplanned direct contact with the study intervention, which may or may not lead to the occurrence of an AE.
>Such persons may include healthcare providers, family members, and other roles that are involved in the trial participant’s care.
>The information must be reported using the Vaccine SAE Report Form.
>Since the information does not pertain to a participant enrolled in the study, the information is not recorded on a CRF.
https://cdn.pfizer.com/pfizercom/2020-11/C4591001_Clinical_Protocol_Nov2020.pdf
Analysis of SARS-CoV-2 spike glycosylation reveals shedding of a vaccine candidate
https://www.biorxiv.org/content/10.1101/2020.11.16.384594v1
Spike protein circulation in the blood post-vaccination
>Circulating Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccine Antigen Detected in the Plasma of mRNA-1273 Vaccine Recipients
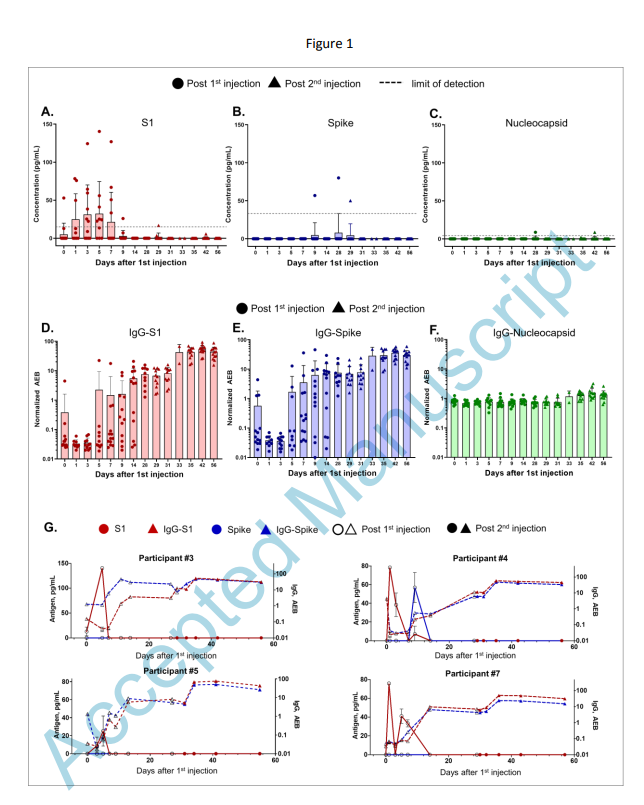
https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab465/6279075#
[Enhanced Respiratory Disease (ERD)? Antibody-Dependent Enhancement (ADE)?]
ERD
>ERD describes severe clinical presentations of respiratory viral infections associated with medical interventions (especially vaccines). Similar clinical presentations can occur as a result of natural infections, and so ERD is detected during preclinical and clinical trials by comparing the distribution of disease severities between the intervention and placebo study arms. ERD can be associated with a broad range of molecular mechanisms, including FcR-dependent antibody activity and complement activation (that is, ADE), but also to other antibody-independent mechanisms such as tissue cell death, cytokine release and/or local immune cell activation.
ADE
>ADE can be broadly categorized into two different types based on the molecular mechanisms involved:
>ADE via enhanced infection. Higher infection rates of target cells occur in an antibody-dependent manner mediated by Fc–FcR interactions. ADE via enhanced infection is commonly measured using in vitro assays detecting the antibody-dependent infection of cells expressing FcγRIIa, such as monocytes and macrophages. The link between in vitro ADE assay results and clinical relevance is often implied, rather than directly observed. Dengue virus represents the best documented example of clinical ADE via enhanced infection.
>ADE via enhanced immune activation. Enhanced disease and immunopathology are caused by excessive Fc-mediated effector functions and immune complex formation in an antibody-dependent manner. The antibodies associated with enhanced disease are often non-neutralizing. ADE of this type is usually examined in vivo by detecting exacerbated disease symptoms, including immunopathology and inflammatory markers, and is most clearly associated with respiratory viral infections. RSV and measles are well-documented examples of ADE caused by enhanced immune activation.
>ERD and ADE (of the second type described above) are often identified by clinical data, including symptom prevalence and disease severity, rather than by the specific molecular mechanisms that drive severe disease. The presence of complex feedback loops between different arms of the immune system makes it very difficult (although not impossible) to conclusively determine molecular mechanisms of ADE and ERD in human and animal studies, even if the clinical data supporting ADE and ERD are quite clear. Many different measurements and assays are used to track ADE and ERD, which can vary based on the specific virus, preclinical and/or clinical protocols, biological samples collected and in vitro techniques used.
>Respiratory ADE is a specific subset of ERD.
https://archive.vn/vvfrv
https://archive.vn/cWgbw
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7569100/
Vaccine researchers, for some reason, don't want to conduct trials/studies or don't want to release detailed data on this and on how it would affect the health of humans who took the vaccine, so there's that.
Also, if you read between the lines, you may notice that often they express no interest in testing for any kind of ADE/VAED/VAERD nor do they have any interest to put any effort to try and purposefully look for any suspicious related data:
>6.2.9. Antibody-dependent enhancement and immunopathology
>The possibility of ADE has also been evaluated in clinical and pre-clinical studies of the vaccines used in this trial.
>Nevertheless, this risk will not have been assessed for heterologous boost schedules.
>Participants will be made aware of this theoretical risk.
"theoretical risk"
>12.9.1. Disease enhancement following vaccination
>Severe COVID-19 disease will be defined as hospitalisation, with further grading of severity according to the WHO ordinal scale (June 2020) (WHO Working Group 2020).
>Cases of COVID19 disease will be examined for the possibility of vaccine associated enhanced disease (VAED).
>This will be evaluated on the basis of the most recent recommendations of the Brighton Collaboration (Brighton Collaboration 2019).
>Samples will be collected for evaluation of immunological evidence of VAED.
>Detailed clinical parameters will be collected from medical records and aligned with agreed definitions, as they emerge.
"aligned with agreed definitions"
https://comcovstudy.org.uk/files/com-cov2protocolv2123-apr-2021cleanpdf
[Available info on ADE with SARS-CoV-2]
In macaques:
The functions of SARS-CoV-2 neutralizing and infection-enhancing antibodies in vitro and in mice and nonhuman primates
>we isolated NAbs against the receptor-binding domain (RBD) and the N-terminal domain (NTD) of SARS-CoV-2 spike from individuals with acute or convalescent SARS-CoV-2 or a history of SARS-CoV-1 infection. Cryo-electron microscopy of RBD and NTD antibodies demonstrated function-specific modes of binding. Select RBD NAbs also demonstrated Fc receptor-γ (FcγR)-mediated enhancement of virus infection in vitro, while five non-neutralizing NTD antibodies mediated FcγR-independent in vitro infection enhancement. However, both types of infection-enhancing antibodies protected from SARS-CoV-2 replication in monkeys and mice. Nonetheless, three of 31 monkeys infused with enhancing antibodies had higher lung inflammation scores compared to controls. One monkey had alveolar edema and elevated bronchoalveolar lavage inflammatory cytokines. Thus, while in vitro antibody-enhanced infection does not necessarily herald enhanced infection in vivo, increased lung inflammation can occur in SARS-CoV-2 antibody-infused macaques
https://www.biorxiv.org/content/10.1101/2020.12.31.424729v2
[]
>ADE in respiratory infections is included in a broader category named enhanced respiratory disease (ERD), which also includes non-antibody-based mechanisms such as cytokine cascades and cell-mediated immunopathology
https://archive.vn/vvfrv
[At least 2 distinct types of ADE]
[1. "Classic", Fc-dependent ADE, as an example noted here for one of the monoclonal antibodies (MW05)]
>results indicate that FcγRIIB is the major FcγR contributing to the enhancement of SARS-CoV-2 infection mediated by MW05
https://www.biorxiv.org/content/biorxiv/early/2020/07/27/2020.07.26.222257.full.pdf
[2. "Unique" to SARS-CoV-2 ADE"]
Type of ADE where antibodies targeting NTD make the NTD stuck to lipid molecules of the cells, enhancing stability, helping the spike come to open conformation (exposing its RBD for better binding) easier
>Antibody dependent enhancement (ADE) of infection is a safety concern for vaccine strategies. In a recent publication, Li et al. (Cell 184 :1-17, 2021) have reported that infection-enhancing antibodies directed against the N-terminal domain (NTD) of the SARS-CoV-2 spike protein facilitate virus infection in vitro, but not in vivo. However, this study was performed with the original Wuhan/D614G strain. Since the Covid-19 pandemic is now dominated with Delta variants, we analyzed the interaction of facilitating antibodies with the NTD of these variants. Using molecular modelling approaches, we show that enhancing antibodies have a higher affinity for Delta variants than for Wuhan/D614G NTDs.
>Using molecular modelling approaches, we show that enhancing antibodies have a higher affinity for Delta variants than for Wuhan/D614G NTDs. We show that enhancing antibodies reinforce the binding of the spike trimer to the host cell membrane by clamping the NTD to lipid raft microdomains. This stabilizing mechanism may facilitate the conformational change that induces the demasking of the receptor binding domain. As the NTD is also targeted by neutralizing antibodies, our data suggest that the balance between neutralizing and facilitating antibodies in vaccinated individuals is in favor of neutralization for the original Wuhan/D614G strain. However, in the case of the Delta variant, neutralizing antibodies have a decreased affinity for the spike protein, whereas facilitating antibodies display a strikingly increased affinity. Thus, ADE may be a concern for people receiving vaccines based on the original Wuhan strain spike sequence (either mRNA or viral vectors). Under these circumstances, second generation vaccines with spike protein formulations lacking structurally-conserved ADE-related epitopes should be considered.
https://www.journalofinfection.com/article/S0163-4453(21)00392-3/fulltext
[]
An infectivity-enhancing site on the SARS-CoV-2 spike protein is targeted by SARS-2 patient antibodies
>we screened a series of anti-spike monoclonal antibodies from COVID-19 patients, and found that some of antibodies against the N-terminal domain (NTD) dramatically enhanced the binding capacity of the spike protein to ACE2, and thus increased SARS-CoV2 infectivity. Surprisingly, mutational analysis revealed that all the infectivity-enhancing antibodies recognized a specific site on the surface of the NTD. The antibodies against this infectivity-enhancing site were detected in all samples of hospitalized COVID-19 patients in the study. However, the ratio of infectivity-enhancing antibodies to neutralizing antibodies differed among patients. Furthermore, the antibodies against the infectivity-enhancing site were detected in 3 out of 48 uninfected donors, albeit at low levels. These findings suggest that the production of antibodies against SARS-CoV-2 infectivity-enhancing site could be considered as a possible exacerbating factors for COVID-19 and that a spike protein lacking such antibody epitopes may be required for safe vaccine development, especially for individuals with pre-existing enhancing antibodies
https://www.biorxiv.org/content/10.1101/2020.12.18.423358v1
Also
Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies
https://archive.vn/vvfrv
>Evidence of ADE in coronavirus infections in vitro
>Findings to date argue against macrophages as productive hosts of SARS-CoV-2 infection
>argue against
*
Monocytes and macrophages, targets of SARS-CoV-2: the clue for SARS-2 immunoparalysis
>SARS-CoV-2 efficiently infects monocytes and macrophages without any cytopathic effect
https://www.biorxiv.org/content/10.1101/2020.09.17.300996v1
*
>“our data show that the presence of an intrauterine bacterial infection results in the infiltration of ACE2 expressing maternal macrophage and neutrophils into and across the placental tissues.These ACE2 expressing immune cells have the potential to transport the virus to the placenta in cases of COVID-19 infection in pregnancy and increase the risk of placental infection and vertical transmission of the virus to the fetus.
https://archive.vn/rODrd
*
>monocytes and macrophages can either be infected by, or phagocytize, SARS-CoV-2
https://www.biorxiv.org/content/10.1101/2020.07.17.209304v1.full.pdf
[Original Antigenic Sin (OAS)?]
OAS - due to the immune system reacting to a somewhat similar pathogen, it does not try to re-adapt, and end up reacting dangerously ineffectively, especially in comparison to how it would react like if it did not encounter a different somewhat similar pathogen before that.
Difference from ADE is that ADE happens only from non-neutralizing antibodies binding to the spike/receptor-binding part part of the virus.
Evidence for Deleterious Original Antigenic Sin in SARS-CoV-2 Immune Response
>Binding assays with patient blood samples directly show cross-reactivity between Abs binding to Ep9 and only one homologous potential antigen, a sequence derived from the neuraminidase protein of H3N2 Influenza A virus. This cross-reactive binding affinity is highly virus strain specific and sensitive to even single amino acid changes in epitope sequence. The neuraminidase protein is not present in the influenza vaccine, and the anti-Ep9 Abs likely resulted from the widespread influenza infection in 2014. Therefore, OAS from a previous infection could underlie some cases of COVID-19 disease severity and explain the diversity observed in disease outcomes.
https://www.biorxiv.org/content/10.1101/2021.05.21.445201v1
[Immune system reprogramming?]
Happens to some extent at least from the mRNA vaccines... Although, essentially it's a downgrade, not remodeling.
The BNT162b2 mRNA vaccine against SARS-CoV-2 reprograms both adaptive and innate immune responses
>These data support the evidence that B.1.351, and possibly other variants, may be able to escape vaccine-induced humoral immunity to a certain extent.
>Intriguingly, the best cellular responses after vaccination were against the B.1.351 variant: the fact that the neutralizing antibody responses against this variant were relatively poor, that may raise the possibility that the protective BNT162b2 vaccine effects against this variant may be mainly reliant on cellular, rather than humoral responses.
>One of the trademarks of trained immunity is an elevated production of inflammatory cytokines following a secondary insult.
>The production of the monocyte-derived cytokines TNF-α, IL-1β and IL-1Ra tended to be lower after stimulation of PBMCs from vaccinated individuals with either the standard SARS-CoV-2 strain or heterologous Toll-like receptor ligands.
>TNF-α production following stimulation with the TLR7/8 agonist R848 of peripheral blood mononuclear cells from volunteers was significantly decreased after the second vaccination.
>Although the concentrations of IFN-α were below the detection limit of the assay for most of the stimuli, we observed a significant reduction in the production if IFN-α secreted after stimulation with poly I:C and R848 after the administration of the second dose of the vaccine.
>This may hamper the initial innate immune response against the virus, as defects in TLR7 have been shown to result in and increased susceptibility to COVID-19 in young males (Van Der Made et 205 al., 2020).
>These results collectively demonstrate that the effects of the BNT162b2 vaccine go beyond the adaptive immune system and can also modulate innate immune responses.
https://www.medrxiv.org/content/10.1101/2021.05.03.21256520v1
[Any additional detailed, sourced info?]
Reminder on the current SARS-CoV-2 vaccines being leaky, as in:
[On Marek's disease]
Scenario of Marek's disease in chicken:
https://archive.vn/W1BdL
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4516275/
https://www.nationalgeographic.com/science/article/leaky-vaccines-enhance-spread-of-deadlier-chicken-viruses
https://en.wikipedia.org/wiki/Marek%27s_disease
https://www.futurity.org/viruses-leaky-vaccines-968692/
https://www.biorxiv.org/content/10.1101/830570v1.full
https://www.healthline.com/health-news/leaky-vaccines-can-produce-stronger-versions-of-viruses-072715
But it's not just chicken, it also has this effect on influenza:
https://www.infectiousdiseaseadvisor.com/home/topics/respiratory/influenza/is-a-leaky-vaccine-mechanism-responsible-to-decreased-flu-season-vaccine-effectiveness/
https://www.contagionlive.com/view/influenza-vaccine-effectiveness-decreases-by-the-season-report-says
BUT!!!
1:1 Marek's disease scenario is quite unlikely to happen due to the vaccinated still being susceptible to the disease from the virus (with its amplification via ADE and failure to respond to infection properly via OAS), what is way more likely is the lethality very slowly crawling up with time (while vaxxed people will still be susceptible and will get a share of health problems both from virus and vaccines), as Marek's disease virus, despite being quite fast at adaptation and mutation, is not as fast as coronaviruses, especially SARS-CoV-2
>Our analysis suggests that meq is estimated to be evolving at a much faster rate than most dsDNA viruses, and is comparable with the evolutionary rate of RNA viruses
https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0162180
Compare pic to https://nextstrain.org/ncov/global
And personally I could not find any reports on MDV shifting epitopes to escape T cell recognition, something that SARS-2 virus was noted and reported to do countless times in various studies and reports.
[Very basic rundown on the mRNA vaccines in general]
https://archive.vn/975Rq
And some detailed info on mRNA vaccines can be found in papers, like in Moderna's mRNA vaccine paper
https://www.nature.com/articles/s41586-020-2622-0.pdf
http://www.freezepage.com/1607356966HPDTGYDFUN
[Human adenovirus-vectored vaccines may be of limited use in the long term, as the human body develops immunity against the adenovirus]
>the presence of preexisting Ad immunity and the rapid development of Ad vector immunity still pose significant challenges to the clinical use of these vectors. Innate inflammatory response following Ad vector administration may lead to systemic toxicity, drastically limit vector transduction efficiency and significantly abbreviate the duration of transgene expression
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)32156-5/fulltext
Pfizer's coronavirus vaccine should be "safe" for most Americans, but 5 groups may want to wait for more data before getting shots
>1.Children under 16
>2.Pregnant people (do they mean pregnant women?)
>3.People who are HIV-positive and others with weakened immune systems must be particularly careful
>4.People with severe allergies to the vaccine's ingredients must hold off
>5.People who previously had COVID-19 are in a gray area
https://archive.vn/RvXx8
>5.People who previously had COVID-19 are in a gray area
In Summer there was an estimate that, compared to official stats, 10x more Americans have been infected
https://archive.vn/xBKnO
[Pfizer leak] [!]
SARS-CoV-2 Vaccine Leaked Data
Russians have hacked into EMA and leaked a bunch of emails and documents
>source
https://0x0.st/-rGv.zip
>password: Moses
>mirror
https://gofile.io/d/1Doara
>unencrypted mirror
https://gofile.io/d/eeKNtb
>alternate mirrors
https://share.dmca.gripe/zRENqooEkvsVP2PO.zip
https://bayfiles.com/v2M1sd9bpe
https://femto.pw/xsx2
https://hitfile.net/v87x3cf
https://turbobit.net/witc0mbhhccl.html
https://pixeldrain.com/u/8tGYy2TD
https://filerio.in/wjok2b6ez58o
https://tusfiles.com/2r0nyxq6l6l6
https://ufile.io/gcr7gfhf
https://www115.zippyshare.com/v/JztupCp6/file.html
https://dl-file.com/kzsjhn663e3w
QRDs (special thanks for anons from /plg/ - Pfizer Leak General https://archive.4plebs.org/pol/search/subject/plg/):
>effectiveness not 95% as it's being marketed
>BNT162b2 when the drug product was stored at the recommended frozen temperature of -70±10 °C for up to 1 month, and accelerated temperatures of 2-8 °C for up to 2 weeks and 25±2 °C for up to 10 days. ('09. Applicant-Rapps-EMA CMC TC - 26.11.2020/Annex 1 - Draft 3.2.P.2.2 Drug Product.pdf' page 20)
>mRNA gets degraded/damaged quickly at room-ambient temperatures outside of freezers, only 76% of the mRNA is left intact in perfect lab conditions before inoculation/getting into the cells through lipid nanoparticles, which, in turn, may possibly result in being translated as trash RNA (as mRNA is compromised in structure). Often it'll be recycled for its components (building blocks) or very rarely will it produce a protein that is misfolded and serves no purpose and eventually be degraded as well
[more on lipid nanoparticles]
tl;dr toxic, inflammatory, may mess up the "timing" of your immune response towards a real infection, resulting in either unproductive response, or a damaging and inflammatory one
The mRNA-LNP platform's lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory
>we present evidence that LNPs used in many preclinical studies are highly inflammatory in mice.
>Intradermal injection of these LNPs led to rapid and robust inflammatory responses, characterized by massive neutrophil infiltration, activation of diverse inflammatory pathways, and production of various inflammatory cytokines and chemokines.
>The same dose of LNP delivered intranasally led to similar inflammatory responses in the lung and resulted in a high mortality rate. In summary, here we show that the LNPs used for many preclinical studies are highly inflammatory.
>Thus, their potent adjuvant activity and reported superiority comparing to other adjuvants in supporting the induction of adaptive immune responses could stem from their inflammatory nature.
>Furthermore, the preclinical LNPs are similar to the ones used for human vaccines, which could also explain the observed side effects in humans using this platform
https://www.biorxiv.org/content/10.1101/2021.03.04.430128v1
https://archive.vn/CJV1h
[]
https://www.researchgate.net/publication/287387193_Development_and_Evaluation_of_Lipid_Nanoparticles_for_Drug_Delivery_Study_of_Toxicity_In_Vitro_and_In_Vivo
https://www.sciencedirect.com/science/article/abs/pii/S0021979720305968
>damaged RNA almost serves no purpose unless it's being broken down into its basic components. There are checklists within the cells that make sure the damaged RNA doesn't get translated into an unusable protein, such as ribosomes not allowing the structure through (no proteins are made), certain regions on the RNA form earlier stop codons (if it does go through the ribosome, the protein being manufactured stops at an early stage), and there are proteins within the cell that proof-read the sequences and if anything's wrong they take care of it (either by disposal or recycling)
>if there was a way the mRNA did produce a functional protein that does something bad there's no way to test for it right now, and that's because there haven't been enough cases with adverse effects to call for that type of testing. To test for that, you'd collect the individuals who did react negatively and simply run protein assays (you compare their protein levels in their blood to a control) and determine what's elevated and what's not. As for the long term effects of the byproducts, I don't think there are any longitudinal studies to warrant any adverse effects since these seem to be novel ingredients made specifically for this vaccine's solution
>plasmacytosis
>liver, lymphatic and nerve inflammation in rat models
>60% enlarged spleen in rat models
>reduced number of mature and immature red blood cells in rat models
>large amount of unclassified cells in blood in rat models, among them:
>1) possibly formation of stem cells in response to organ trauma
>2) possibly also LARGE unspecified cells. Large cells can mean a few things (people refer to ovum as large cells as they are visible with the naked eye as well as being the largest cell in the body phagocytes are also large cells though): large cell is a term used in oncology, it does not refer to a particular type of cell; rather it refers to cells that are larger than would be normally expected for that type. It is frequently used when describing lymphoma and lung cancer. The phrase giant cell is also frequently used, especially with carcinoma. The leaked paper does not go into any greater detail describing these "large cells" so no definitive conclusions can be drawn for now
>surfactants in the vaccine solution may be the cause of inflammation (these are triggering your immune system and other organ systems as a result):
>ALC-0315 (acetamide side-chain in ALC-0315 could be a possible culprit in the unspecified cell formation, since acetamides are known carcinogens) are there to prevent the solution from clumping in on itself, ensuring everything in the vaccine solution remains 'itself'. These two components have an interesting metabolic cycle (how they get degraded in the body) where they must first undergo degradation in the liver (and this is why there's enlargement of livers and associated organs/tissue (spleens, bone marrow, immune cell count)) in which the by-product is turning out to be somewhat toxic (however, this was not common)
>ALC-0159 (more concerning because of the biotransformations it undergoes. Since this compound has an acetamide moiety (side group) and it is known to be a carcinogen, it may be the cause of the adverse side-effects the studies are seeing because of the metabolic pathways it undergoes. Also, ALC-0159 is shown to be more stable in your body with a 120min mark (>82% remaining) in liver microsomes and 240min (>87% remaining) in hepatocytes, and that's probably why we're seeing toxicity within the liver and associated organ/tissue systems))
>carcinogenous metabolite that needs further investigation
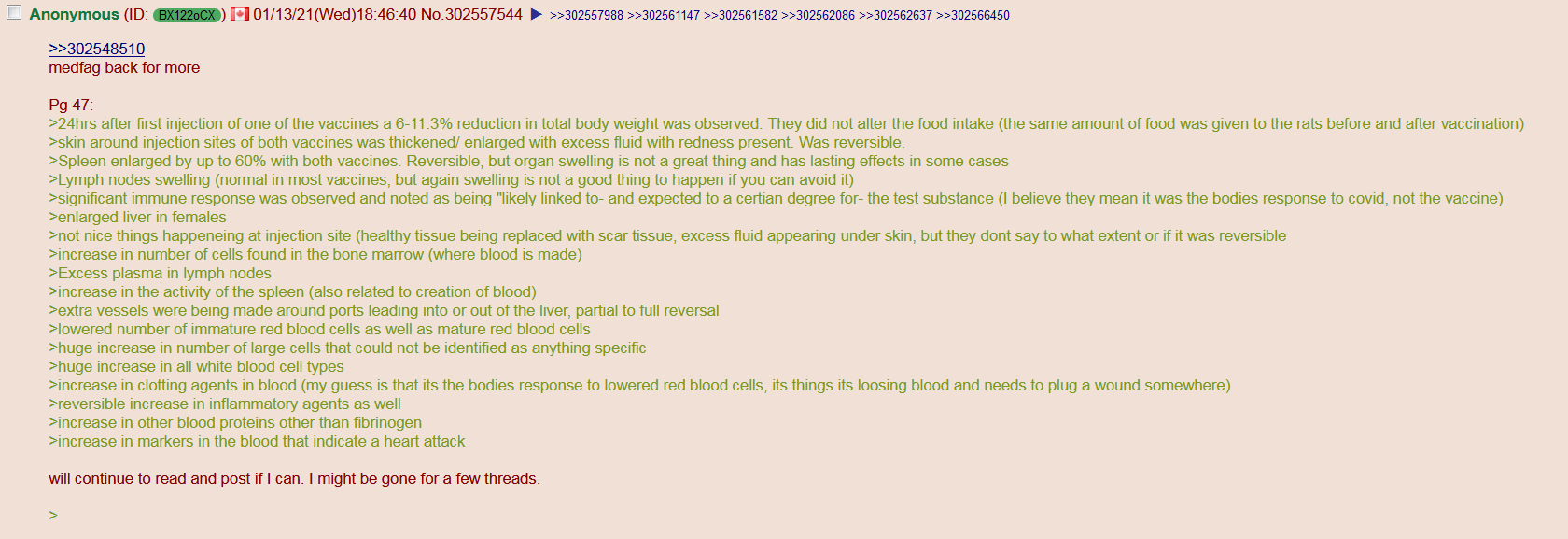


EMA quality review of toxicology (page 46)


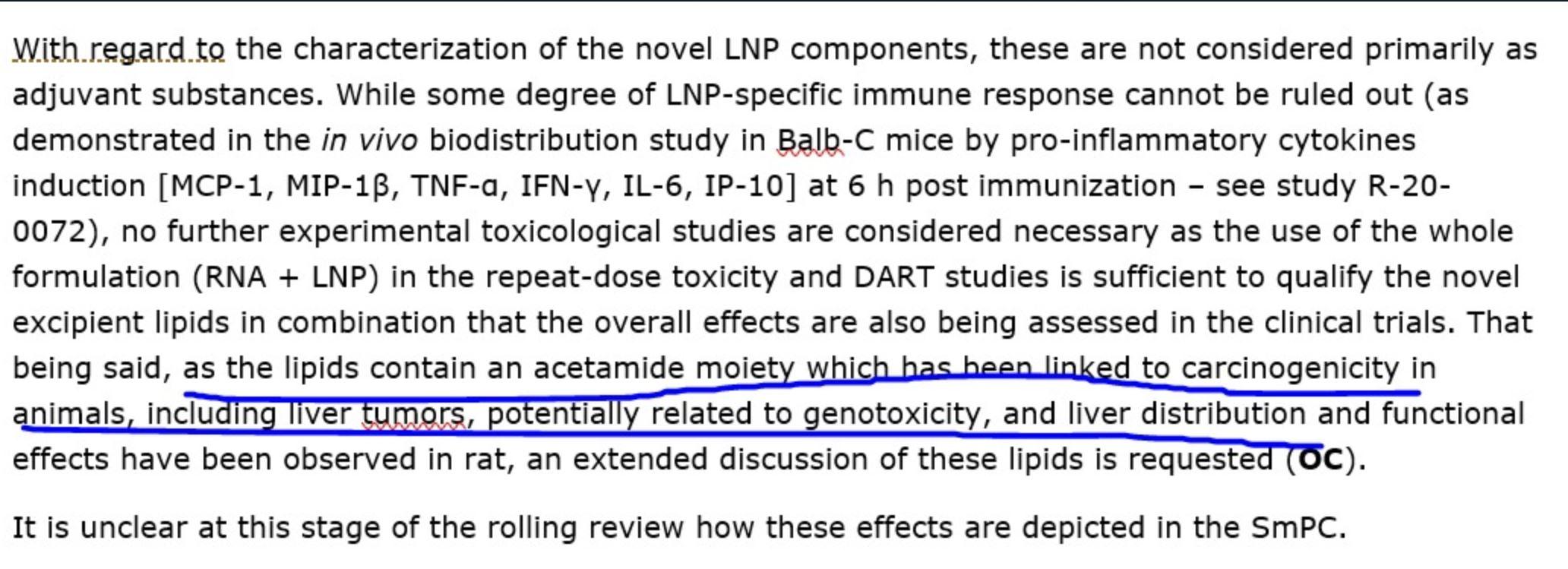
[pfizer leak - moar]
https://www.slideshare.net/enave2609/ss-242999249

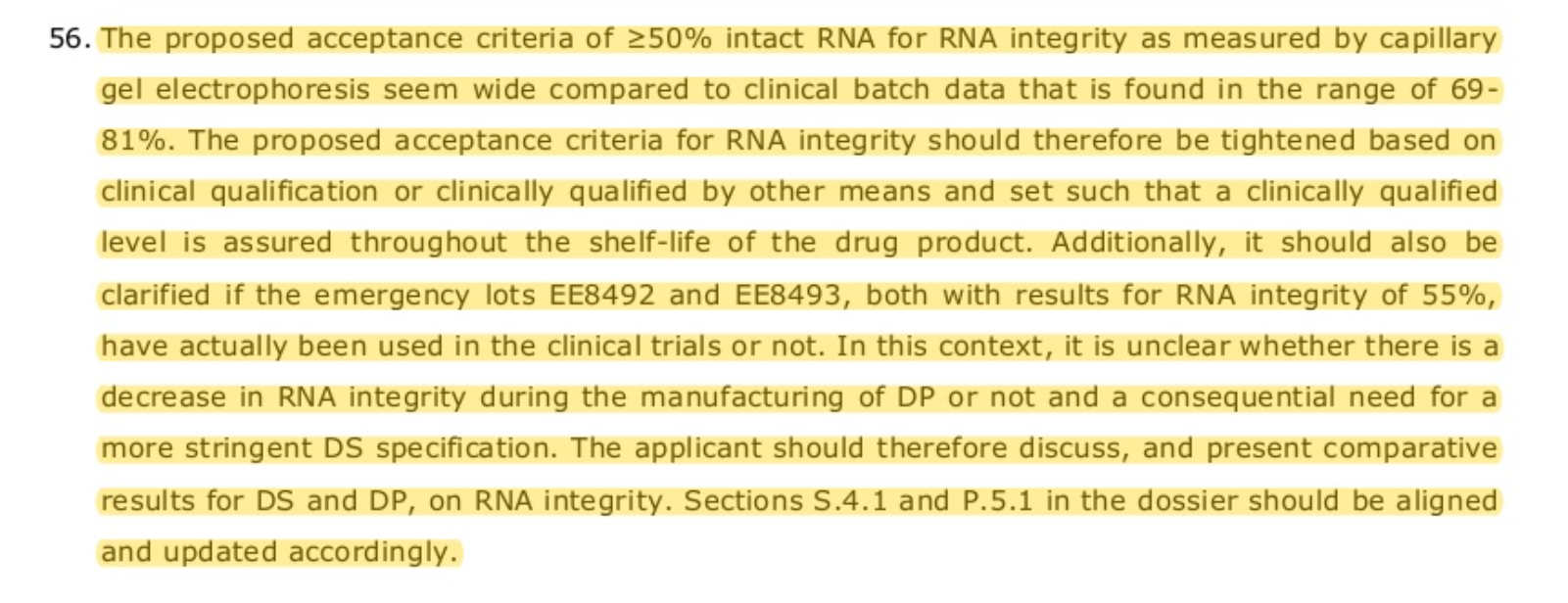
Zero clues on how stable their mRNA, aside from the fact that the payload may be degraded up to 50%
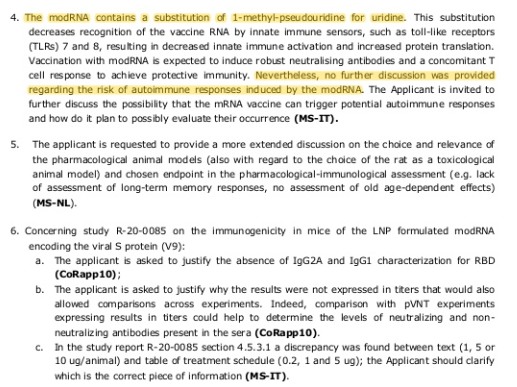
>risk of autoimmune problems from modRNA - brought up
>needed research and data to assess safety - not

>re-presenting the spike triggers the inflammation
>re-presenting the RBD does not
Almost like the RBD is not of natural origin and is there to stay in the virus, with it not aggravating the pathology too much and not being susceptible to become deleted/changed

Accumulation of surfactans in the liver (and other organs maybe?)

Lipid nanoparticles overstimulating inflammation


Toxicity and accumulation of surfactants in the liver, again
[If you want to be vaccinated with something with known risks, safety, and at least some actual efficacy, then consider getting a BCG shot and/or a polio shot and/or an MMR shot and/or a tetanus shot and/or a diphteria shot instead]
RCT for BCG vaccination against SARS-2
>elderly Greek patients were randomized (1:1) to receive either BCG revaccination or placebo at hospital discharge, followed by 6 months observation for incidence of COVID19 infection.
>BCG revaccination resulted in 68% risk reduction for total COVID19 clinical and microbiological diagnoses (OR 0.32, 95% CI 0.13-0.79).
>Five patients in the placebo group and one in the BCG-vaccinated group had severe COVID19 that necessitated hospitalization. 3 months after BCG vaccination 1.3% of placebo and 4.7% of BCG-vaccinated volunteers had anti-SARS-CoV-2 antibodies.
>These data argue that BCG revaccination is safe and protects the elderly against COVID19. BCG revaccination may represent a viable preventive measure against COVID19.
https://www.medrxiv.org/content/10.1101/2021.05.20.21257520v1
BCG vaccination confers protection from SARS-2 via cross-reactive SARS-CoV-2-specific T cell responses
https://archive.vn/6umvh
https://www.news-medical.net/?tag=/BCG-Vaccine
BCG vaccine demonstrates effectiveness after 40 years (cross-reactivity with SARS-CoV-2 would last ~5 years after (re)vaccination)
https://archive.vn/V1YVf
[]
>Since the delayed cell-mediated immune response, historically measured as immunoreactivity to tuberculin (tuberculin sensitivity test or TST) and presumably, the concomitant “trained immunity” conferred by BCG vaccination at childbirth wanes rapidly within first few years of childhood in the absence of “booster” Mycobacterium spp. exposures [12,15,16], the prevalent actual “trained immunity” and heterologous cell-mediated immunity correlate of any population could be a better evaluable predictive parameter of populations’ response to COVID-19 infections
https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1008969
Reactions to the BCG vaccine are uncommon and generally mild.
>The most common side effects include fever, headache and swollen glands.
>More serious complications, such as abscesses or bone inflammation, are rare.
>Most children develop a sore at the injection site. Once healed, the sore may leave a small scar. This is normal and nothing to worry about.
https://www.nhs.uk/conditions/vaccinations/bcg-tb-vaccine-side-effects
BCG revaccination boosts adaptive polyfunctional Th1/Th17 and innate effectors in IGRA+ and IGRA– adults
https://archive.vn/g8FHo
No cross-reactivity from BCG-induced antibodies with SARS-CoV-2, thus no chance of ADE effect from it:
Common childhood vaccines do not elicit a cross-reactive antibody response against SARS-CoV-2
>we tested whether BCG, Pneumococcal, Rotavirus, Diphtheria, Tetanus, Pertussis, Hepatitis B, Haemophilus influenzae, Hepatitis B, Meningococcal, Measles, Mumps, and Rubella vaccines provide cross-reactive neutralizing antibodies against SARS-CoV-2 in BALB/c mice. Results indicated that none of these vaccines provided antibodies capable of neutralizing SARS-CoV-2 up to seven weeks post vaccination. We conclude that if such vaccines have any role in COVID-19 immunity, this role is not antibody-mediated.
https://archive.vn/d2vB3
Vaccination against tuberculosis has influence on the epidemiology of SARS-2
>analysis of statistical data was conducted by experts from St Petersburg University
>incidence of COVID-19, the course of acute interstitial pneumonia caused by infection, and the mortality rate from it are associated with being vaccinated with bacilli Calmette-Guerin (BCG) according to the national vaccination schedule. The mortality rate turned out to be lower in those countries and areas where national vaccine immunization programmes have taken place for a long time or continue today, especially if revaccinations were practiced.
>BCG vaccine activates a local immune response on the mucous membranes. It is through them that acute respiratory disease caused by SARS-CoV-2 spreads
>BCG vaccine serves as a trigger for a 'trained' immune system response that activates monocytes, macrophages and natural killer cells - that power in the non-antigen-specific protective rogrammes of the body. Also, gamma-interferon, produced after BCG vaccination, and other mediators may ultimately attenuate the course of COVID-19.
>'The causative agent of the new coronavirus infection and BCG have common peptides, which means that induction of cross-immunity is possible. Large clinical trials of the BCG vaccine and trials of its use for the prevention of the new coronavirus infection are currently underway, for example, in the Netherlands and Australia'
https://www.news-medical.net/news/20201203/Vaccination-against-tuberculosis-has-influence-on-the-epidemiology-of-COVID-19.aspx
>BCG vaccination could reduce the infection caused by SARS-2 and MCV2 vaccine years increases the total infection rate. This study identified high economic characteristics and not having universal BCG coverage as the independent risk factors of mortality by the multivariate analysis.
https://pubmed.ncbi.nlm.nih.gov/33588980/
Trained immunity’ from Mycobacterium spp. (environmental or BCG) exposure predicts protection from SARS-2
https://www.medrxiv.org/content/10.1101/2021.02.11.20233593v1
BCG Vaccine Derived Peptides Induce SARS-CoV-2 T Cell Cross-Reactivity
>we identify 8 novel BCG-derived peptides with significant sequence homology to either SARS-CoV-2 NSP3 or NSP13-derived peptides. Using an in vitro co-culture system, we show that human CD4+ and CD8+ T cells primed with a BCG-derived peptide developed enhanced reactivity to its corresponding homologous SARS-CoV-2-derived peptide. As expected, HLA differences between individuals meant that not all persons developed immunogenic responses to all 8 BCG-derived peptides. Nevertheless, all of the 20 individuals that were primed with BCG-derived peptides developed enhanced T cell reactivity to at least 7 of 8 SARS-CoV-2-derived peptides
https://www.frontiersin.org/articles/10.3389/fimmu.2021.692729/full
Effect of BCG vaccination on proinflammatory responses in elderly individuals
>We investigated the influence of Bacillus Calmette-Guérin (BCG) vaccination on the unstimulated plasma levels of a wide panel of cytokines, chemokines, acute-phase proteins (APPs), matrix metalloproteinases (MMPs), and growth factors in a group of healthy elderly individuals (age, 60 to 80 years) at baseline (before vaccination) and 1 month after vaccination as part of our clinical study to examine the effect of BCG on COVID-19. Our results demonstrated that BCG vaccination resulted in diminished plasma levels of types 1, 2, and 17 and other proinflammatory cytokines and type 1 interferons. BCG vaccination also resulted in decreased plasma levels of CC, CXC chemokines, APPs, MMPs, and growth factors. Plasma levels of the aforementioned parameters were significantly lower in vaccinated individuals when compared to unvaccinated control individuals. Thus, our study demonstrates the immunomodulatory properties of BCG vaccination and suggests its potential utility in nonspecific vaccination of COVID-19 by down-modulating pathogenic inflammatory responses.
https://advances.sciencemag.org/content/7/32/eabg7181
https://archive.is/9hBDE
Study shows BCG vaccine can protect mice against lethal SARS-CoV-2 challenged
https://www.news-medical.net/news/20210901/Study-shows-bacille-Calmette-Guerin-vaccine-can-protect-mice-against-lethal-SARS-CoV-2-challenge.aspx
Poliovirus vaccination induces an adaptive humoral immune response. Antibodies (anti-D3-pol) created by poliovirus vaccination bind the RNA-dependent RNA polymerase (RdRp) protein of both poliovirus and SARS-CoV-2, thereby preventing SARS-CoV-2 infection
>Countries with effective poliovirus immunization protocols and younger populations have fewer and less pathogenic cases of COVID-19. Antibodies to poliovirus and SARS-CoV-2 were found in pediatric sera and in sera from adults recently immunized with polio. Sera from polio-immunized individuals inhibited SARS-CoV-2 infection of Vero cell cultures.
https://www.frontiersin.org/articles/10.3389/fmed.2021.710010
Diphtheria and/or tetanus shots helping against SARS-2
>individuals with registered diphtheria or tetanus vaccinations were less likely to develop severe COVID-19 than people who had only received other vaccinations (diphtheria OR=0.46, p=3.6×10−4; tetanus OR=0.50, p=5.8×10−4)
https://www.medrxiv.org/content/10.1101/2021.06.09.21257809v1
Measles/mumps/rubella (MMR) shots helping against SARS-2
>The OR for testing positive in recently vs not recently MMR-vaccinated was 0.91.
>OR in males was 0.43, suggesting a protective effect in males but not females.
https://www.sciencedirect.com/science/article/pii/S0264410X21007957
Influenza and/or pneumococcal vaccine
>The researchers found that the patients who had been vaccinated for influenza had lower odds of symptomatic disease than the patients who hadn’t been vaccinated (adjusted odds ratio [aOR]=0.714, 95% CI [0.529, 0.964], p=0.028). A similar effect was seen in patients who had been vaccinated for the pneumococcal vaccine (aOR=0.482, 95% CI [0.277, 0.837], p=0.010).
https://www.contemporarypediatrics.com/view/did-flu-shots-and-pneumococcal-vaccines-offer-some-covid-19-protection
Warning - there's always a possibility of a new influenza virus strain surfacing, with possibly making your disease worse if you had the flu shot, like it happened multiple times in the past:
>2008-9 flu shot doubled the risk of illness from the 2009 H1N1 pandemic flu (Skowronski, PLoS Medicine 2010); the increased H1N1 illness risk in US children associated with the 2015-16 nasal flu vaccine (Jackson, NEJM 2017) ; and the 68% increase in H3N2 illness risk among UK elderly after the 2016-17 flu shot (Pub Health England 8/31/17).
https://www.bmj.com/content/359/bmj.j5759/rr
>Supplementary food for thought
[You're considered to be not fully vaccinated within first two weeks after your latest SARS-2-vaccination]

https://www.cdc.gov/coronavirus/2019-ncov/vaccines/fully-vaccinated.html
[C.D.C. Will Not Investigate Mild Infections in Vaccinated Americans]
At least 10,000 vaccinated people were infected with the coronavirus through the end of April. Now the agency has stopped pursuing the mildest cases.
>Through the end of April, when some 101 million Americans had been vaccinated, the C.D.C. had received 10,262 reports of breakthrough infections from 46 states and territories, a number that was very likely “a substantial undercount,” according to a C.D.C. report issued on Tuesday.
>Critics say the agency is missing important opportunities to learn about the real-world effectiveness of the different vaccines and to gather information that might help identify trends in the pandemic’s trajectory — for example, how long vaccine protection lasts, or how various vaccines compare in preventing infection with variants, or whether certain patients like older people are more susceptible to breakthrough infections.
>“We are driving blind, and we will miss a lot of signals,” said Ali Mokdad, an epidemiologist at the University of Washington who spent many years as a senior scientist at the C.D.C.
>“The C.D.C. is a surveillance agency,” Dr. Mokdad said. “How can you do surveillance and pick one number and not look at the whole?”
>The change was announced quietly in a statement on the agency’s website this month. It said the switch “will help maximize the quality of the data collected on cases of greatest clinical and public health importance.”
>Another rationale given for not tracking all breakthrough infections is that they are not likely to result in further spread of the virus.
https://archive.is/OHkE5
https://web.archive.org/web/20210525201004/https://www.nytimes.com/2021/05/25/health/cdc-coronavirus-infections-vaccine.html
[At least in USA, "breakthrough", aka "cases of infection within the vaccinated individuals" are being "counted" via a criteria that would allow for only anomalous and extremely high-viral-load cases to be counted as positive]
tl;dr
>Unvaccinated person taking PCR test - cycle threshold value at 35 or even 40 (depends on how much you need to inflate the numbers). Despite having no symptoms, you can easily become a “covid case”.
>Vaccinated person taking PCR test - cycle threshold value at 28 or lower because “possible breakthrough infections” (whatever the fuck that means). Harder to test positive and you have to end up in hospital or literally die in order to count as a covid infection.
CDC page
https://archive.is/8Uwaj
Not tl;dr
Average PCR kit cycle threshold for normal unvaccinated cases are the following:
>FDA GenePro2 usage manual
>Ct Value Analysis
>The cut-off Ct value for all target genes is 31.
>If RNA from each sample has the Ct value lower than 31, it is regarded as viral RNA isolation is passed, while if the Ct value is equal to or higher than 31, it is regarded as viral RNA isolation is failed.
https://www.fda.gov/media/139442/download
[]
>TABLE 1: SARS-COV-2 MOLECULAR ASSAY EVALUATION: RESULTS
https://www.finddx.org/covid-19/sarscov2-eval-molecular/molecular-eval-results/
https://www.finddx.org/wp-content/uploads/2020/07/FIND_SARS-COV2_molecular-assay-evaluation-results_03Jul2020.pdf
But, in USA, for breakthrough cases, way higher standards to count them as a confirmed cases are used:
>COVID-19 vaccine breakthrough case investigation
>Specimen selection
>Clinical specimens for sequencing should have an RT-PCR Ct value ≤28.
>If a Ct value is not available, specimens that are positive for SARS-CoV-2 RNA or antigen by another testing modality may be sent.
[In order to encourage American workers to get vaccinated, the Occupational Safety and Health Administration has suspended the legal requirement for employers to report work-related injuries resulting from SARS-2 vaccinations]
>In order to encourage American workers to get vaccinated, the Occupational Safety and Health Administration (OSHA) has suspended the legal requirement for employers to report work-related injuries resulting from vaccinations aimed at combating the CCP virus
>employers may be still held liable under workers’ compensation laws or under civil personal injury laws
>in May, website of OSHA, an agency within the U.S. Department of Labor (DOL), stated that employers could be held liable if they required employees to receive COVID-19-related injections as a condition of employment and the employees then experience adverse reactions.
>visitors to the same website’s FAQ section now see a message, which reads:
>“DOL and OSHA, as well as other federal agencies, are working diligently to encourage COVID-19 vaccinations. OSHA does not wish to have any appearance of discouraging workers from receiving COVID-19 vaccination, and also does not wish to disincentivize employers’ vaccination efforts.
>As a result, OSHA will not enforce 29 CFR 1904’s recording requirements to require any employers to record worker side effects from COVID-19 vaccination through May 2022.
>We will reevaluate the agency’s position at that time to determine the best course of action moving forward.”
>“No doubt receiving pressure from the Biden administration, OSHA suspended the enforcement requirement to record adverse injuries or death from COVID shots until May 2022 in order to push the COVID shots. This politically motivated change by OSHA is unprecedented,” Liberty Counsel group stated in a press release
https://archive.is/CSv4j
[Australia - Collating vaccination status of cases is not NSW's priority, says CHO]
>By Mary Ward
>Every day, NSW Chief Health Officer Kerry Chant has been asked about the vaccination status of the state's ICU cases.
>While this data is published for overall cases in NSW Health's weekly surveillance report, a specific breakdown of the status of cases in hospitals and ICU has not been provided.
>The Chief Health Officer said that while NSW Health's epidemiological staff would try to pull together that data, it was not their priority given the amount of information available abroad.
>"Can I just reassure the community: we don't need to look at our own data to know that the vaccines are effective," Dr Chant said.
https://archive.is/G4Vg6
[Pfizer chief says people will probably require yearly SARS-CoV-2 vaccine booster shot]
DESPITE:
[Reanalysis of the Pfizer mRNA BNT162b2 SARS-CoV-2 vaccine data fails to find any increased efficacy following the boost: Implications for vaccination policy and our understanding of the mode of action]
>A non-weighted three-segment, two knot linear regression was fitted to the published cumulative infection incidence from the Pfizer BNT162b2 vaccine Phase III trial using the lspine routine in R.
>The optimal knot days were estimated through sensitivity analysis and the confidence limits for efficacy estimates were determined by Monte Carlo Simulations.
>This analysis showed the vaccine was effective from day 11 post first vaccination.
>The estimated efficacy over the period 11 to 28 days post first vaccination was 0.94 and there was no detectable increase in efficacy following the second vaccination.
>The efficacy post first vaccination substantially preceded the development of detectable serum neutralizing antibody.
>Strongly protective immunity develops rapidly following a single vaccination and at least in the short period covered by the timetable of the Phase III trial, there was no additional benefit from a second vaccination.
>This increases options for use of this vaccine, e.g., for ring fence vaccination, for use in travelers and for mass vaccination rollout. It highlights the need for further research into duration of immunity following a single vaccination and for understanding mechanisms of protection.
https://www.medrxiv.org/content/10.1101/2021.02.23.21252315v1
[Pharmaceutical corporations given vaccine liability exemptions from governments]
https://archive.vn/21ORQ
https://archive.vn/zxgzM
https://archive.vn/qzPfO
https://archive.vn/WJ4gK
https://archive.vn/eM6Pv
You can’t sue Pfizer or Moderna if you have severe SARS-CoV-2 vaccine side effects. The government likely won’t compensate you for damages either
>Under the PREP Act (USA), companies like Pfizer and Moderna have total immunity from liability if something unintentionally goes wrong with their vaccines.
>A little-known government program provides benefits to people who can prove they suffered serious injury from a vaccine
>That program rarely pays, covering just 29 claims over last 10 years
https://archive.vn/OsjS6
>while the pandemic continues, Moderna will not enforce our COVID-19 related patents against those making vaccines intended to combat the pandemic
https://archive.is/602G4
CEO of Pfizer says everyone should "trust science" and to get the vaccine because its your loved ones that will pay for it if you don't.
>He was then asked why he hasn't had the vaccine yet. He said "he's also 59 and in relatively good health, so it's not entirely appropriate for him to receive the vaccine before other people who "need it more" ". If he was vaccinated on camera, he said "it might help increase the public's willingness to receive it", citing Pfizer's internal research. But he emphasized that "none of the executives and board members will cut the line."
https://archive.vn/ti56o
[Governments' and pharmaceutical corporations' vaccine agreement leaks]
[Israel government, Pfizer lead illegal experiment on humans]
> On January 6, the “Cooperation Agreement for the Collection of Epidemiological Information from the Real World” was signed, in which Pfizer promised the State of Israel vaccines that would vaccinate all residents except children and those who could not be vaccinated. In return for advancing Israel over other countries, the State of Israel will provide Pfizer with epidemiological information that will allow it to assess the effectiveness of the vaccine.
> The question that arose in the Israeli public was: what medical information the State of Israel would be given to Pfizer? What exactly would it be used for?
> Many allegations have been made about the risk of violating the privacy of the country’s residents, and the lack of transparency in not disclosing the contract. The Ministry of Health said that it would give Pfizer only general epidemiological information, but then why did Pfizer sign an agreement to obtain such information, which is exposed in any case. The Ministry of Health agreed to publish the full agreement. However, parts in the agreement are hidden under black marks. Why?
[]
>Israeli scientist, the agreement between Pfizer and the government is that no adverse events from the vax are to be disclosed for a minimum of 10 years.
>“Ithe agreement that countries had with Pfizer does not allow them to escape their contract, which states that even if a drug will be found to treat COVID-19, the contract cannot be voided.”
https://archive.is/AsP4X
[]
[Pfizer-Albania contract leak]

https://web.archive.org/web/20210727075553/https://twitter.com/eh_den/status/1419660536657154059
https://web.archive.org/web/20210726214053/https://gogo.al/wp-content/uploads/2021/01/LEXO-KONTRATEN-E-PLOTE.pdf
https://web.archive.org/web/20210727080201/https://gogo.al/ekskluzive-kontrata-sekrete-e-qeverise-me-pfizer-per-vaksinat
https://files.catbox.moe/hk1vn0.pdf
[]
[Pfizer-Brazil contract leak]
https://theswissbay.ch/pdf/Politics/contrato-pfizer-SIGNED.pdf
[Developers of Oxford-AstraZeneca Vaccine Tied to UK Eugenics Movement]
>The developers of the Oxford-AstraZeneca vaccine have previously undisclosed ties to the re-named British Eugenics Society as well as other Eugenics-linked institutions like the Wellcome Trust.
https://archive.vn/UPMiQ
https://archive.is/i4qq4
[Oxford Kept SARS-2 Vaccine Trial Volunteers in Dark About Dosing Error, Letter Shows]
https://archive.vn/JB9HQ
-
Johnson & Johnson has lost major lawsuits in 1995, 1996, 2001, 2010, 2011, 2016, 2019 (for what it's worth, J&J's vaccine also contains tissues from aborted fetal cells, perhaps a topic for another discussion)
-
Pfizer has the distinction of the biggest criminal payout in history. They have lost so many lawsuits it's hard to count. You can check out their rap sheet here. Maybe that's why they are demanding that countries where they don't have liability protection put up collateral to cover vaccine-injury lawsuits. Also check https://www.corp-research.org/pfizer
-
Astra Zeneca has similarly lost so many lawsuits it's hard to count. Here's one. Here's another.. And here's a full pack https://www.corp-research.org/astrazeneca And in case you missed it, the company had their SARS-2 vaccine suspended in at least 18 countries over concerns of blood clots, and they completely botched their meeting with the FDA with numbers from their study that didn't match (as if that would apply only to AstraZeneca)
-
J&J (whose vaccine is approved for "Emergency Use" in the US) and Astrazenca (whose vaccine is not approved for "Emergency Use" in the US), had a little mix up in their ingredients...in 15 million doses.
[Trial dates of the vaccines distributed for emergency]
Also AstraZeneca/ChadOx won't finish phase 2 until October 2021
https://clinicaltrials.gov/ct2/show/NCT04324606
And Moderna will not count phase 3 as complete until October 2022.
https://www.clinicaltrials.gov/ct2/show/NCT04470427
[As of September 2021, The Pfizer vaccine is still extended under the EUA Emergency Use Authorization]
Read slowly, second paragraph on page 2, slowly, understand the trickery in the wording.
The Pfizer vaccine is still extended under the EUA Emergency Use Authorization. This means the vaccine company nor the US gubmint can be liable for your harm, death, siezures, medical bills ect. The "green light" is for the Biotech Cormirnaty vaccine.
>EUA will remain in place for the Pfizer-BioNTech COVID-19 vaccine for the previously-authorized indication and uses, and to authorize use of COMIRNATY (COVID-19 Vaccine, mRNA) under this EUA for certain uses that are not included in the approved BLA
>Pfizer-BioNTech COVID‑19 Vaccine contains a nucleoside-modified messenger RNA (modRNA) encoding the viral spike (S) glycoprotein of SARS-CoV-2 formulated in lipid particles. COMIRNATY (COVID-19 Vaccine, mRNA) is the same formulation as the PfizerBioNTech COVID-19 Vaccine and can be used interchangeably with the Pfizer-BioNTech COVID-19 Vaccine to provide the COVID-19 vaccination series.
>The Pfizer vaccine is still extended under the EUA Emergency Use Authorization. This means the vaccine company nor the US gubmint can be liable for your harm, death, siezures, medical bills ect. The "green light" is for the Biotech Cormirnaty vaccine.
Page 12 is the real nitty gritty. First paragraph. Says all that you need to know.
Page 12, second paragraph and third paragraph, you realize that Pfizer and Biotech are two companies, the Cormirnaty vaxx is the new drug that is FDA approved and they also state that some, not all, but some of the Pfizer vaccines are manufactured with the same standards as the BioTech vaccine.
>All descriptive printed matter, advertising, and promotional material relating to the use of the Pfizer-BioNTech COVID‑19 Vaccine clearly and conspicuously shall state that:
>This product has not been approved or licensed by FDA, but has been authorized for emergency use by FDA, under an EUA to prevent Coronavirus Disease 2019 (COVID-19) for use in individuals 12 years of age and older; and
>The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of the medical product under Section 564(b)(1) of the FD&C Act unless the declaration is terminated or authorization revoked sooner.
https://www.fda.gov/media/150386/download
[]
>Under this license, you are authorized to manufacture the product, COVID-19 Vaccine, mRNA, which is indicated for active immunization to prevent coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 16 years of age and older.
>You may label your product with the proprietary name, COMIRNATY, and market it in 2.0 mL glass vials, in packages of 25 and 195 vials
Meanwhile scroll down:

"Post-approval" seems to be "post-COMIRNATY-approval" (in reference to previous post) while "BNT162b2" is still continuing testing what is just in Emergency Use Authorization but physically/chemically is the same as what was approved
https://www.fda.gov/media/151710/download
[Funding, shareholders and connections]
The top five individual shareholders of Johnson and Johnson include CEO, Alex Gorsky—the largest shareholder with 2.9 million shares.
https://en.wikipedia.org/wiki/Alex_Gorsky
Joaquin Duato—J&J's executive vice president—is the second-largest individual shareholder with 916,576 shares.
https://youtube.com/watch?v=U85gyFDmbl8
Paulus Stoffels, Ph.D., is the third-largest individual shareholder with 739,236 shares. Stoffels is JNJ’s chief scientific officer.
https://en.wikipedia.org/wiki/Paul_Stoffels
Jennifer Taubert is a leader within J&J's pharmaceutical research division and is the fourth-largest shareholder with 402,592 shares.
https://khn.org/news/pharma-execs-dig-in-for-a-fight-against-outraged-senators/
The fifth-largest individual shareholder of J&J is CFO, Joseph J. Wolk with 84,997 shares.
https://kobi5.com/news/johnson-johnson-cfo-says-vaccine-outweighs-risks-149144/
These people, Johnson & Johnson along with AstraZeneca, Pfizer, Moderna, Bill and Mel Gates, Hospitals, Doctor's, Nurses, Armed forces, Religious representatives, Politicians, Governments, Lobbyists, Media companies, Social-Media companies etc. can not be held liable or sued for any injuries or deaths caused by the snake oil as they have all washed their hands with the people of the world.
FactCheck.org is funded by the Robert Wood Johnson foundation which holds 15.9% (now around 22.4%) of its assets largely benefitting from Johnson and Johnson's success with the vaccine.
The website has become a central source for fact checking, debunking and promoting COVID-19 news and information all while stating it’s unbiased
>Robert wood Johnson holdings
https://www.holdingschannel.com/top/stocks-held-by-robert-wood-johnson-foundation/
> SciCheck’s COVID-19/Vaccination Project is made possible by a grant from the Robert Wood Johnson Foundation.
https://www.factcheck.org/covid-misconceptions/
>The president and CEO of the Robert Wood Johnson Foundation is Richard E. Besser, who was the former acting director of the Centers for Disease Control and Prevention (CDC) and chief health and medical editor at ABC News.
https://bigleaguepolitics.com/factcheck-orgs-covid-19-project-is-funded-by-foundation-that-has-15-percent-of-its-assets-in-johnson-and-johnson-stock/
Good job on reading it all.