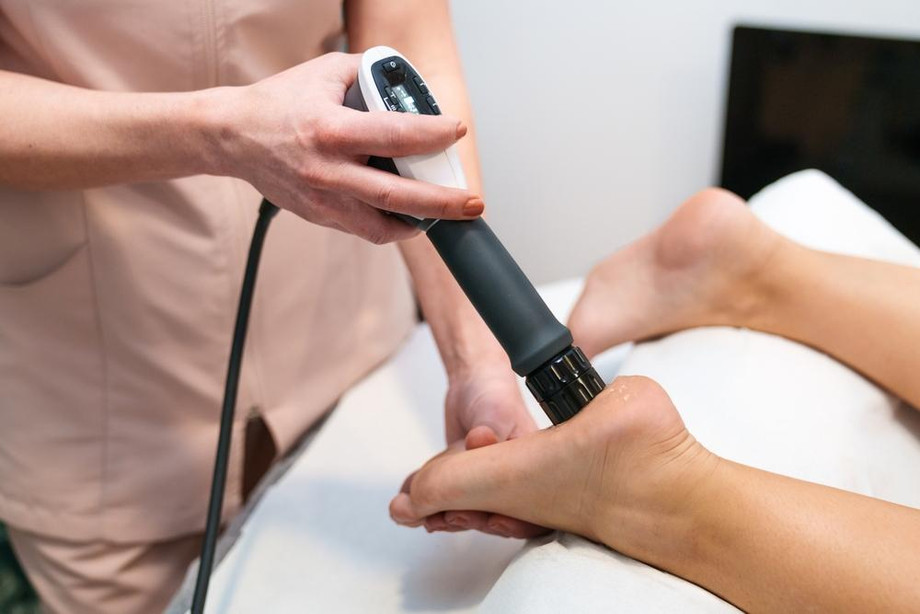
Over the last decade, for non-invasive pain relief devices has surged, driven by an aging population, rising prevalence of chronic pain conditions, and growing awareness of alternatives to opioid therapy. Healthcare providers and patients alike are turning to technologies that deliver targeted analgesia without surgical intervention or systemic side effects. Key growth drivers include increasing adoption of wearable electrotherapy systems, portable ultrasound units, and transcutaneous electrical nerve stimulation (TENS) devices. Economies with expanding healthcare infrastructure are witnessing robust uptake of non-invasive solutions, as reimbursement policies evolve to cover innovative therapies. Investors and equipment manufacturers are capitalizing on these trends, engaging in joint ventures, mergers, and product launches to capture share in both established and emerging. Enhanced focus on improving patient adherence through smartphone connectivity and user-friendly interfaces is further accelerating expansion. Stakeholders monitoring shipment volumes and sales revenue of leading suppliers will note that regions such as North America, Europe, and Asia Pacific are setting benchmarks in unit installations, penetration, and revenue growth for non-invasive pain management technology.
Technological Innovations Leading Non-Invasive Pain Relief Solutions
Breakthroughs in microcurrent stimulation, pulsed electromagnetic field therapy (PEMF), and focused ultrasound are revolutionizing the pain management landscape. Microcurrent devices deliver sub-sensory electrical currents to modulate cellular repair pathways, offering relief for musculoskeletal pain and nerve injuries. PEMF systems use low-frequency electromagnetic waves to reduce inflammation and promote tissue healing. Meanwhile, wearable ultrasound patches and handheld devices enable point-of-care thermal ablation or deep-tissue stimulation without invasive procedures. Integration of artificial intelligence and data analytics within these platforms is fostering personalized therapy regimens based on real-time feedback and usage patterns. Haptic sensors embedded in ergonomic wearables track patient movement and adjust stimulation parameters dynamically, ensuring optimal therapeutic dosing. Research labs continue to refine device footprints, battery life, and signal fidelity, catering to home-use patients and ambulatory settings.
Clinical Applications and Patient-Centered Outcomes
Non-Invasive Pain Management Devices are gaining traction across a spectrum of indications—from chronic lower back pain and osteoarthritis to postoperative discomfort and neuropathic syndromes. Clinical studies demonstrate that regular use of TENS units and PEMF mats can yield significant reductions in pain intensity scores, improved joint mobility, and decreased reliance on analgesic medication. In postoperative recovery, low-intensity ultrasound therapy has been shown to expedite wound healing, minimize scar formation, and alleviate surgical site tenderness. Patients with diabetic peripheral neuropathy report enhanced sensory function and pain relief when using remote-controlled microcurrent patches in conjunction with standard care. The shift toward patient-centered outcomes emphasizes quality-of-life metrics, such as sleep quality, physical activity levels, and overall satisfaction with non-pharmacological pain management. Insurance providers are increasingly covering device rental or purchase costs when medical necessity is established, further legitimizing non-invasive solutions. Comparative effectiveness research continues to explore head-to-head modalities and combination protocols, highlighting potential synergies between electrical, thermal, and mechanical stimulation therapies.
Regulatory Landscape Shaping Device Approvals and Standards
Regulatory bodies worldwide maintain stringent pathways for authorization of non-invasive pain management devices, ensuring safety, efficacy, and quality control. Class II medical devices often require submission of clinical data demonstrating non-significant risk profiles and substantial equivalence to predicate devices. Adherence to ISO 13485 quality management systems and good manufacturing practices is mandatory for global distribution. In certain jurisdictions, additional post- surveillance and adverse event reporting obligations apply, particularly for novel technologies such as high-intensity focused ultrasound. Labeling requirements clear instructions on intended use, contraindications, and warnings to mitigate misuse. Regulatory harmonization efforts under organizations like the International Medical Device Regulators Forum (IMDRF) are streamlining submission dossiers, aiding multinational manufacturers. For stakeholders evaluating product pipelines and approval timelines, tracking regulatory clearances, 510(k) summaries, and CE mark certifications offers insight into competitive positioning. Understanding regional regulatory nuances, such as local clinical evaluation reports or bench testing mandates, is critical for successful entry and ongoing compliance.
Commercial and Transactional Opportunities for Industry Stakeholders
The competitive landscape for non-invasive pain management solutions is characterized by aggressive product differentiation, strategic partnerships, and licensing agreements. Leading device manufacturers continually update their portfolios via next-generation offerings, incorporating features like Bluetooth connectivity, smartphone apps, and modular electrode designs. Contract manufacturing organizations and component suppliers are scaling up production capacities to meet surging , while distributors expand their service networks to include training, maintenance, and consultancy services. From a transactional standpoint, participants engage in contract negotiations for bulk orders, service-level agreements, and distribution rights. Pricing strategies vary—from outright device purchase models to subscription-based access for bundled hardware and software services. Investment funds and venture capital firms target companies demonstrating strong intellectual property portfolios, clinical validation, and recurring revenue streams. For procurement managers and hospital administrators seeking to compare supplier catalogs, request quotations, or negotiate volume discounts, vendor directories and trade shows remain invaluable. Online places also facilitate direct ordering of FDA-cleared TENS units, PEMF mats, and ultrasound therapy devices, streamlining the purchase process for clinics and home users alike.
Get This Report in Japanese Language -非侵襲性疼痛管理デバイス市場
Get This Report in Korean Language -비침습적 통증 관리 장치 시장
Read More Articles Related to this Industry –
Nanofiber Applications in Medical Devices: Revolutionizing Healthcare
Camera Modules in Medical Devices: Revolutionizing Diagnostics and Treatment
About Author:
Priya Pandey is a dynamic and passionate editor with over three years of expertise in content editing and proofreading. Holding a bachelor's degree in biotechnology, Priya has a knack for making the content engaging. Her diverse portfolio includes editing documents across different industries, including food and beverages, information and technology, healthcare, chemical and materials, etc. Priya's meticulous attention to detail and commitment to excellence make her an invaluable asset in the world of content creation and refinement.
(LinkedIn- https://www.linkedin.com/in/priya-pandey-8417a8173/)