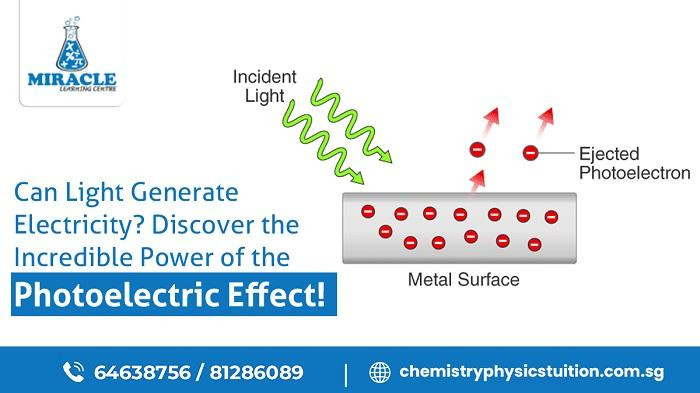
The photoelectric effect has captured the attention of scientists for over a century. This phenomenon, discovered by Albert Einstein, revolutionized our understanding of light and laid the foundation for quantum theory. In simple terms, the photoelectric effect is the emission of electrons from a material when it is exposed to light. But the implications of this process go far beyond its initial observation. For students eager to not only grasp the concepts but also excel in their understanding, joining a physics tuition program is a wise decision. In this detailed study, we'll dig into the details of the photoelectric effect and uncover the secrets that are central to quantum mechanics.
History of the Photoelectric Effect
The photoelectric effect has a rich and intriguing history that dates back to the late 19th century. It all began when Heinrich Hertz observed the phenomenon of radio waves, which led to the discovery of electromagnetic radiation. This newfound knowledge ignited a fervor among scientists, eager to uncover the true nature of light.
However, it was Albert Einstein who made the groundbreaking connection between light and the emission of electrons. In 1905, Einstein published a paper that presented his theory on the photoelectric effect, for which he would later be awarded the Nobel Prize in Physics. This discovery challenged the prevailing belief that light behaved purely as a wave and introduced the concept of light as discrete packets of energy, known as photons.
Key Concepts of the Photoelectric Effect
To understand the photoelectric effect, it is essential to grasp a few key concepts. Firstly, the energy of a photon is directly proportional to its frequency. This means that higher frequency light, such as ultraviolet or X-rays, carries more energy than lower frequency light, such as infrared or radio waves.
Secondly, every material has a specific threshold frequency, also known as the work function. If the incoming photons have a frequency below this threshold, no electrons will be emitted, regardless of the intensity of the light. However, if the frequency exceeds the threshold, electrons will be liberated, and their kinetic energy will depend on the difference between the energy of the incident photons and the work function.
Experimental Evidence for the Photoelectric Effect
Numerous experiments have provided solid evidence for the existence of the photoelectric effect. One such experiment was conducted by Philipp Lenard, who observed that the intensity of light did not affect the kinetic energy of emitted electrons but rather the number of electrons ejected. This phenomenon could not be explained by the prevailing wave theory of light and provided further support for Einstein's particle theory.
Another crucial experiment was carried out by Robert Millikan, who measured the charge on individual electrons using his famous oil-drop experiment. By combining his findings with Einstein's theory, Millikan confirmed the relationship between the energy of photons and the kinetic energy of emitted electrons, validating the concepts behind the photoelectric effect.
Einstein's Explanation of the Photoelectric Effect
Einstein's explanation of the photoelectric effect was a turning point in the history of physics. He proposed that light is composed of discrete particles, or photons, each carrying a specific amount of energy. When these photons strike the surface of a material, they transfer their energy to electrons within the material. If the energy of the photons exceeds the work function, the electrons gain enough energy to overcome the attractive forces holding them in place and are emitted as photoelectrons.
This revolutionary theory explained several puzzling observations, such as the instantaneous emission of electrons when light is incident on a material, as well as the dependence of the photoelectric current on the intensity and frequency of light. Einstein's explanation also provided a foundation for the development of quantum mechanics, which revolutionized our understanding of the microscopic world.
Applications of the Photoelectric Effect
The photoelectric effect has a wide range of practical applications that have greatly impacted various fields of science and technology. One of the most prominent applications is in solar cells, which harness the photoelectric effect to convert sunlight into electricity. Solar cells consist of semiconductor materials that absorb photons and generate an electric current as a result of the photoemission of electrons.
Another notable application is in night vision technology. Night vision devices, such as goggles or cameras, utilize the photoelectric effect to amplify the intensity of incoming light. These devices employ specialized materials that emit electrons when exposed to photons, creating an electron cascade that amplifies the original signal, enabling enhanced vision in low-light conditions.
Conclusion
Through a combination of historical discoveries, experimental evidence, and theoretical breakthroughs, we have unravelled the mysteries of how light can both act as a particle and a wave. The photoelectric effect has paved the way for advancements in quantum mechanics and has contributed to our modern technological landscape. And, if you want to learn more about physics and dive into the interesting world of quantum mechanics, it's a good idea to join a physics tuition in Singapore. These specialized programs provide structured learning environments and expert guidance, fostering a deeper appreciation for the complexities of the subject. So, the next time you turn on a light or admire the sun's strength, think about how important the photoelectric effect is and how it helps us understand the universe better.