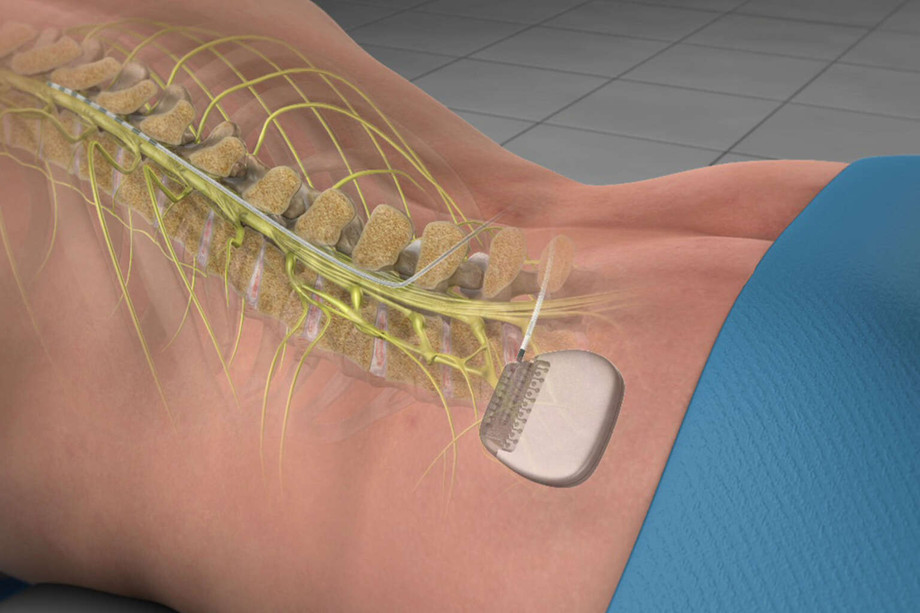
Emerging Technologies Transforming Spinal Non Fusion Treatments
Spinal non fusion devices represent a paradigm shift in the management of degenerative spine disorders, trauma, and deformities. These implants, designed to stabilize the spine without the need for permanent bony fusion, rely on flexible materials, dynamic stabilization systems, and motion-preserving mechanisms to maintain near-natural kinematics. Early generations of these devices focused primarily on interspinous process spacers, aiming to alleviate nerve compression by limiting extension. More recent innovations incorporate elastomeric cores, polyetheretherketone (PEEK) constructs, and titanium alloys engineered for customized flexibility profiles. The use of 3D-printed architectures and advanced surface coatings enhances osseointegration and reduces micromotion, potentially lowering hardware-related complications. With growing clinical evidence supporting their ability to maintain segmental mobility, researchers continue to develop next-generation solutions that combine load sharing, shock absorption, and real-time motion feedback.
Clinical Benefits and Patient Outcomes of Non Fusion Spine Devices
Patients treated with a Spinal Non Fusion Device, such as dynamic stabilization or total disc replacement systems, often see quicker recovery and less adjacent segment degeneration compared to traditional fusion. By preserving motion, these devices reduce stress on nearby vertebrae and improve outcomes like pain (VAS) and disability (ODI) scores. They also lower the need for bracing, shorten hospital stays, and reduce revision surgeries. Long-term data shows sustained relief and strong device durability. As patient selection improves, Spinal Non Fusion Devices are becoming a preferred solution for maintaining spinal function.
Competitive Landscape Overview
The global for minimally invasive and motion-preserving spine technologies is fueled by an aging population, increasing prevalence of chronic back pain, and rising preference for outpatient procedures. Cost analyses reveal that dynamic stabilization procedures can offer a cost-effective alternative by reducing indirect expenses related to rehabilitation and work absenteeism. Key drivers include expanding indications for total disc replacement, growing awareness of adjacent segment disease, and favorable reimbursement policies in several regions. Leading medical device companies continue to invest heavily in research and development, acquiring emerging players who hold proprietary polymers or sensor-enabled platforms. Mergers and strategic alliances are reshaping the competitive arena, with tier-1 suppliers focusing on expanding product portfolios and geographic reach.
Regulatory Landscape and Approval Updates
Navigating the regulatory environment is critical for successful commercialization of spinal non fusion products. The approval pathway for these devices varies by jurisdiction, encompassing rigorous preclinical biomechanical testing, clinical investigations, and post surveillance requirements. Recent updates from major regulatory authorities have clarified guidelines on cyclic fatigue testing, wear debris analysis, and long-term safety monitoring. In specific regions, expedited review programs for breakthrough technologies have enabled time-to- acceleration, provided robust evidence of substantial clinical benefit. Real-world data collection through registries and digital health platforms is increasingly leveraged to satisfy postapproval follow-up obligations. As regulatory bodies emphasize patient-reported outcomes and value-based assessments, manufacturers are incorporating health economics and outcomes research (HEOR) into their submissions.
Access and Navigate to the Latest Comprehensive Spine Device Report
To gain a holistic understanding of the spinal non fusion device landscape, stakeholders are encouraged to access the latest detailed analysis. The report overview features segmentation by device type, end-user, and geography, combined with supply chain intelligence on key raw material sources. Readers can navigate to the report section dedicated to clinical trial pipelines and patent filings, which sheds light on emerging innovations and licensing agreements. An interactive dashboard within the report allows for scenario modeling based on projected procedure volumes and pricing trends. By exploring downloadable insights in the research overview, users can tailor the data to their strategic priorities, whether evaluating competitive positioning or identifying high-growth s. This navigation tool ensures a seamless pathway from high-level drivers to granular vendor profiles and financial forecasts.
How to Purchase or Download Detailed Insights on Non Fusion Spine Devices
For those seeking a transactional engagement, the report provides options to purchase individual chapters or the complete document bundle. Pricing tiers accommodate academic institutions, small-medium enterprises, and corporate clients, with license terms permitting multi-user access and periodic updates. Prospective buyers can order a sample executive summary that highlights key findings, methodology, and forecast assumptions. Upon purchase, an email with secure download links grants immediate access to the full suite of data tables, graphical analyses, and expert commentary. Custom research services are also available, allowing for tailored analyses such as white label reports or bespoke sizing exercises. Payment options include credit card, purchase order, and wire transfer, ensuring a flexible procurement process for decision-makers in life sciences, investment banking, and supply chain management.
Spinal non fusion devices are poised to capture an increasing share of the overall spine implant , driven by continuous technological advancements and rising patient for motion preservation. Emerging opportunities lie in combining smart materials with embedded sensors to enable remote monitoring of implant performance, patient activity levels, and early detection of complications. Collaborations with digital health platforms and artificial intelligence algorithms could further tailor postoperative care and enhance long-term outcomes. On the commercial front, expanding reimbursement frameworks in emerging economies and the growing adoption of value-based care models in established healthcare systems will stimulate broader uptake. entrants focusing on cost-competitive, regionally adapted product lines may gain traction in underserved s, while established players pursue strategic acquisitions to bolster their dynamic stabilization portfolios. A comprehensive, navigable research report remains an indispensable resource for charting the competitive dynamics, investment hotspots, and regulatory trajectories shaping the future of spinal non fusion therapies.
Get This Report in Japanese Language - 脊椎非固定デバイス市場
Get This Report in Korean Language - 척추 비융합 장치 시장
Read More Articles Related to this Industry –
Nanofiber Applications in Medical Devices: Revolutionizing Healthcare
Camera Modules in Medical Devices: Revolutionizing Diagnostics and Treatment
About Author:
Priya Pandey is a dynamic and passionate editor with over three years of expertise in content editing and proofreading. Holding a bachelor's degree in biotechnology, Priya has a knack for making the content engaging. Her diverse portfolio includes editing documents across different industries, including food and beverages, information and technology, healthcare, chemical and materials, etc. Priya's meticulous attention to detail and commitment to excellence make her an invaluable asset in the world of content creation and refinement.
(LinkedIn- https://www.linkedin.com/in/priya-pandey-8417a8173/)