what are covalent compounds?
Introduction:-
what are covalent compounds? is our main discussion.Today I will explain on a nice topic of chemistry,what are covalent compounds? Hope this will help a lot for school children of class (ix) and class(x).
Explanation:-
To start today's discussion let, us first know
What are elements?
Answer:- The matters which are composed of only one type of particles are known as elements.e.g; Sodium (Na), Magnesium(Mg), Carbon(C).....
What are atoms?
Answer:- The smallest part of an element
which is unbreakable chemically are known as atoms.
e.g; Sodium(Na) ,Helium(He), Lithium(Li) ,all are monoatomic elements. Oxygen(O₂) is a diatomic molecule.Water(H₂O) is a triatomic molecule.
What are molecules?
Answer:- When two or more than two similar or different types of atoms combine together chemically they form molecules
e.g;
O + O —------------------> O₂
Oxygen molecule(diatomic)
2H₂ + O₂—------------------> 2H₂O
Water molecule(triatomic)
What are compounds?
Abswer:- When two or more molecules or atoms chemically react themselves under some definite conditions and combines then they form compounds.
e.g;
N₂ + 3H₂ —----------------> 2NH₃
(molecule) (molecule) (Ammonia is a compound)
C + O₂—---------------------->CO₂
(atom) (molecule) (carbon dioxide is a gas/compound)
Now we will learn our main topic
what are covalent compounds?
Answer:- The compounds which are formed by the mutual sharing of electrons between two non metal atoms, are known as covalent compounds.
e.g; Methane(CH₄),Ethene(C₂H₄),Ethyne(C₂H₂) etc.
Now I will discuss additionally,
How methane(CH₄) is formed?
Answer:- Electronic configuration of carbon is 2,4 ; To complete octet and get its nearest noble gas neon(Ne) gas configuration(2,8) and to become stable carbon need 4more electrons or to get duplet and get helium gas(He) gas configuration (2)carbon need to give up its 4 valence shell electrons but carbon atom is too small due to its inter molecular electronic force of attraction carbon atom neither able to give up 4 valence nor it can get 4 electrons from other atoms.
On the other hand hydrogen atom is very small and have only 1 valence electrons which occupies its first shell(s-shell) to get its nearest noble gas helium (He),hydrogen atom need one more electron but it is unable to gain one electron from other atoms.
So, 4 hydrogen ato-
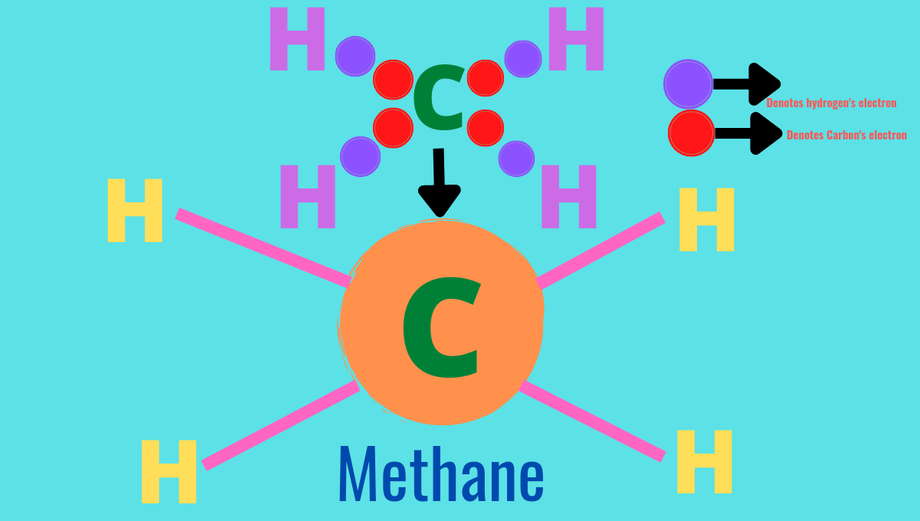
ms share their lone electron each with one carbon forming four covalent single bonds and thus methane(CH₄) is formed. The formation can be given as:-
To Read...

