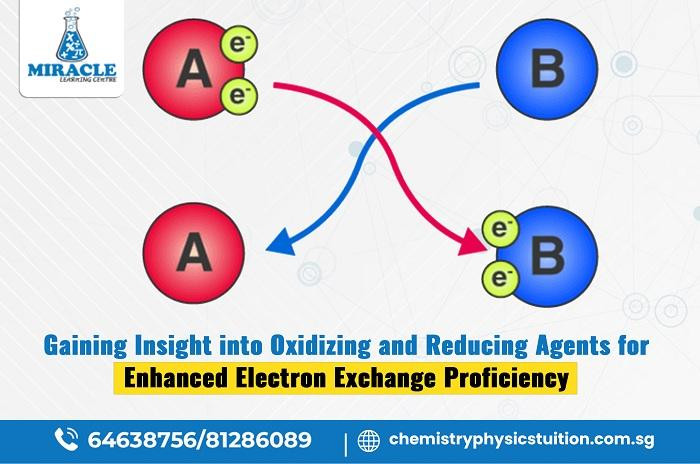
Within the intricate landscape of chemistry, the roles of oxidizing and reducing agents stand as pivotal players in the orchestration of chemical reactions. These agents wield the power to either accept or donate electrons, steering transformations within substances. Understanding their significance is paramount in comprehending the nuances of chemical processes. Oxidizing agents, avid electron acquirers, contrast sharply with reducing agents, known for their propensity to donate electrons. Studying electron transfer in chemistry tuition reveals the essence of these agents, showcasing their roles in redox reactions. Mastering these concepts unlocks the secrets of chemical transformations, deepening understanding in the chemical realm.
Oxidizing Agents: The Electron Acquirers
An oxidizing agent, also known as an oxidant or oxidizer, is a compound that facilitates oxidation in other compounds. During oxidation, the oxidizing agent accepts electrons from another compound, termed the reducing agent. This electron acceptance leads to the oxidizing agent's reduction, where its oxidation state decreases due to gaining electrons.
This process can manifest in various ways, such as the oxidizing agent releasing oxygen to the other compound, receiving hydrogen from it, or directly accepting an electron without involving oxygen or hydrogen exchange.
Examples of oxidizing agents include:
- Oxygen
- Ozone
- Halogens like Fluorine and Chlorine
- Sulfuric acid
For instance, in the combustion of methane (CH4), oxygen (O2) acts as an oxidizing agent. Oxygen accepts electrons from methane, resulting in the formation of carbon dioxide (CO2) and water (H2O). This reaction showcases the oxidizing properties of oxygen, facilitating the oxidation of methane to its oxidized products.
Reducing Agents: Electron Donors
A reducing agent operates in the opposite direction of an oxidizing agent. It functions by reducing other compounds through the donation of electrons to the oxidizing agent, thereby undergoing oxidation itself in the process. Remember, the oxidizing agent undergoes reduction by accepting electrons, while the reducing agent gets oxidized by donating electrons.
The oxidation of a reducing agent can happen in various ways: it might accept oxygen from another compound, release hydrogen to another compound, or provide an electron to another compound without exchanging oxygen or hydrogen. This leads to an increase in the oxidation state of the reducing agent.
Reducing agents are sometimes referred to as reducers or reductants. Examples of reducing agents include:
- Sodium (Na)
- Magnesium (Mg)
- Aluminum (Al)
- Hydrogen (H2)
- Cuprous ion (Cu+)
These substances exhibit reducing properties by donating electrons to facilitate chemical reactions.
How to Identify Oxidizing and Reducing Agents?
Steps to Identify:
- Identify Oxidation States: Determine the initial and final oxidation states of elements involved in the reaction.
- Recognize Changes: Note any increase or decrease in oxidation states during the reaction.
- Oxidation vs. Reduction: Elements with increased oxidation states are oxidized (acting as reducing agents), while those with decreased oxidation states are reduced (acting as oxidizing agents).
Examples:
In a reaction involving the combustion of methane (CH4) with oxygen (O2), follow these steps:
Identify Oxidation States:
- Methane (CH4) has carbon with an oxidation state of -4 and hydrogen with +1.
- Oxygen (O2) has an oxidation state of 0.
Recognize Changes:
- In the reaction, carbon in methane changes from -4 to +4 (CO2), while oxygen in O2 changes from 0 to -2 (CO2).
- Carbon undergoes an increase in oxidation state (from -4 to +4), indicating oxidation.
- Oxygen undergoes a decrease in oxidation state (from 0 to -2), indicating reduction.
Oxidation vs. Reduction:
- Methane (CH4) acts as a reducing agent as carbon is oxidized.
- Oxygen (O2) acts as an oxidizing agent as it is reduced.
This exemplifies how methane (reducing agent) is oxidized by oxygen (oxidizing agent) in a redox reaction, emphasizing the transfer of electrons between species.
Balancing Chemical Equations
Balancing redox equations requires careful consideration of electron transfer. Using oxidation numbers and the half-reaction method, the equation is balanced by ensuring the conservation of mass and charge throughout the reaction.
Applications of Oxidizing and Reducing Agents
Oxidizing and reducing agents find diverse applications across industries and daily life, influencing crucial processes:
- Bleaching Agents: Oxidizing agents like chlorine and hydrogen peroxide are used as bleaching agents in the textile and paper industries.
- Corrosion Control: Reducing agents such as zinc and cadmium are used to prevent corrosion in metals by acting as sacrificial anodes.
- Water Treatment: Chlorine-based oxidizing agents are utilized for disinfection and purification of water in municipal treatment plants.
- Chemical Synthesis: Both oxidizing and reducing agents are critical in the synthesis of various chemicals and pharmaceuticals.
These agents play vital roles in bleaching, corrosion prevention, water purification, and chemical synthesis, showcasing their significance in multiple sectors.
The Significance of Chemistry Tuition to learn it
The foundation of knowledge in chemistry is the understanding of oxidising and reducing agents. Getting the best chemistry tuition in Singapore allows you to work with knowledgeable tutors in the field.
These specialised programmes help students understand the complex ideas of electron transfer in redox reactions by providing them with in-depth knowledge, problem-solving strategies, and real-world applications. Chemistry tuition fosters a deep appreciation for these essential chemical processes in addition to improving comprehension.
Students can successfully navigate these ideas with individualized attention and knowledgeable guidance, laying a solid foundation for future investigation into the field of chemistry. Selecting the best chemistry tuition in Singapore guarantees a thorough comprehension and proficiency of the functions of reducing and oxidising agents in chemical reactions.
Bottom Line
Oxidizing and reducing agents are fundamental in chemical reactions, driving essential processes in various industries and everyday life. Their roles in electron transfer significantly influence the outcome of redox reactions. Through comprehensive understanding and guidance provided by chemistry tuition, individuals can navigate these concepts effectively, laying a strong foundation for further exploration and application in the diverse realms of chemistry.