Dr. Anthony Fauci got into a heated argument with Sen. Rand Paul this week over the origins of the coronavirus.
In fact, he lied to Congress saying the U.S. government never funded Gain-of Function research... Oh, but it has, and I'll show the clear proof.
That's not all dear reader we here at anonunknownymous have now for over a year been screaming about the very things Benn has highlighted , he does it because Fauci is his target we did it because we see a smoking gun
Ben Swann
14 May 2021
The NIH Director December 19, 2017 NIH Lifts Funding Pause on Gain-of-Function Research
NIH Lifts Funding Pause on Gain-of-Function Research
Today, the National Institutes of Health announced that it is lifting a funding pause dating back to October 2014 on gain-of-function (GOF) experiments involving influenza, SARS, and MERS viruses. GOF research is important in helping us identify, understand, and develop strategies and effective countermeasures against rapidly evolving pathogens that pose a threat to public health. The funding pause was lifted in response to today’s release of the
' Department of Health and Human Services Funding Decisions about Proposed Research Involving Enhanced Potential Pandemic Pathogens ' <(link is external) <(HHS P3CO Framework).
The HHS P3CO Framework describes a multi-disciplinary review process, involving the funding agency and a Department-level review group, that considers the scientific merits and potential enefits of the research, as well as the potential to create, transfer, or use an enhanced potential pandemic pathogen. This framework formalizes robust oversight for federally funded research with enhanced pathogens of pandemic potential. It is the product of an extensive deliberative process undertaken by experts throughout the public and private sectors,and is aligned with the Recommended Policy Guidance for Departmental Development of Review Mechanisms for Potential Pandemic Pathogen Care and Oversight (P3CO)(link is external).
We have a responsibility to ensure that research with infectious agents is conducted responsibly, and that we consider the potential biosafety and biosecurity risks associated with such research. I am confident that the thoughtful review process laid out by the HHS P3CO Framework will help to facilitate the safe, secure, and responsible conduct of this type of research in a manner that maximizes the benefits to public health.
I would especially like to acknowledge the efforts of the National Science Advisory Board for Biosecurity and the National Academies of Sciences, Engineering, and Medicine(link is external) for their thoughtful deliberations on the issues surrounding this important area of research. The work of these committees was instrumental in guiding the United States Government in its job of creating rigorous policy that allows vital research to move forward.
Francis S. Collins, M.D., Ph.D.
Director, National Institutes of Health
Related Links
Statement on Funding Pause on Certain Types of Gain-of-Function Research
Statement on the HHS framework to guide funding for avian (HPAI) H5N1 influenza research
https://grants.nih.gov/grants/guide/notice-files/NOT-OD-17-071.html
Notice Number: NOT-OD-17-071
Key Dates
Release Date: December 19, 2017
NOT-OD-15-011
Issued by
National Institutes of Health (NIH)
Purpose
The purpose of this Guide Notice is to notify applicants that in accordance with the December 2017 issuance of the Department of Health and Human Services "HHS Framework for Guiding Funding Decisions about Proposed Research Involving Enhanced Potential Pandemic Pathogens (HHS P3CO Framework),” the National Institutes of Health is removing the funding pause on the provision of new or continuation funding for gain-of-function research projects.
Background
On October 17, 2014, the U.S. Government announced that it would be instituting a funding pause on gain-of-function research projects that could be reasonably anticipated to confer attributes to influenza, MERS, or SARS viruses such that the resulting virus has enhanced pathogenicity and/or transmissibility (via the respiratory route) in mammals. During the funding pause, the U.S. Government undertook a deliberative process to assess the potential benefits and risks associated with these types of studies. Completion of the deliberative process resulted in the Department of Health and Human Services issuing the HHS P3CO Framework on December 19, 2017. The HHS P3CO Framework is responsive to and in accordance with the "Recommended Policy Guidance for Departmental Development of Review Mechanisms for Potential Pandemic Pathogen Care and Oversight" issued on January 9, 2017 and supersedes the previous "Framework for Guiding U.S. Department of Health and Human Services Funding Decisions about Research Proposals with the Potential for Generating Highly Pathogenic Avian Influenza H5N1 Viruses that are Transmissible among Mammals by Respiratory Droplets.”
The HHS P3CO Framework is intended to guide agency funding decisions on proposed research that is reasonably anticipated to create, transfer, or use enhanced potential pandemic pathogens (PPPs). A PPP is any pathogen that satisfies both of the following:
It is likely highly transmissible and likely capable of wide and uncontrollable spread in human populations; and
It is likely highly virulent and likely to cause significant morbidity and/or mortality in humans.
An enhanced PPP is defined as a PPP resulting from the enhancement of the transmissibility and/or virulence of a pathogen.
The HHS P3CO Framework ensures a multidisciplinary, department-level pre-funding review and evaluation of proposed research meeting the scope of the framework. The HHS P3CO Framework can be viewed HERE.
Impact
The HHS P3CO Framework impacts research with any pathogen that meets the definition of enhanced PPP. NIH will continue to accept applications for research projects including studies that are reasonably anticipated to create, transfer or use enhanced PPPs. As described in the background section of this Guide Notice, NIH will identify meritorious research applications meeting the scope of the HHS P3CO Framework for a multidisciplinary, department-level review prior to any funding decision.
If a Scientific Review Group (SRG) identifies research that may create, transfer, or use enhanced PPPs as described above, the Scientific Review Officer will record this as an administrative note and instruct the SRG members that the presence of such research in the application will not affect their impact scores. Following completion of the SRG review, Program officials will review all proposed research being considered for funding to determine if the research meets the scope of the HHS P3CO Framework, and if it does, will work with the applicant, institution, and funding agency staff, as appropriate, to comply with the HHS P3CO Framework.
Inquiries
Please direct all inquiries to the Program Official that is listed in the Funding Opportunity Announcement or the Notice of Award under the Contacts Section.
Article Info Publication History Published: February 2018 Identification DOI: https://doi.org/10.1016/S1473-3099(18)30006-9
Access this article on ScienceDirect https://web.archive.org/web/20210517001341/https://www.pnas.org/content/113/11/3048SARS-like WIV1-CoV poised for human emergence
See all authors and affiliations
-
Edited by Peter Palese, Icahn School of Medicine at Mount Sinai, New York, NY, and approved January 6, 2016 (received for review September 4, 2015)
Abstract
Outbreaks from zoonotic sources represent a threat to both human disease as well as the global economy. Despite a wealth of metagenomics studies, methods to leverage these datasets to identify future threats are underdeveloped. In this study, we describe an approach that combines existing metagenomics data with reverse genetics to engineer reagents to evaluate emergence and pathogenic potential of circulating zoonotic viruses. Focusing on the severe acute respiratory syndrome (SARS)-like viruses, the results indicate that the WIV1-coronavirus (CoV) cluster has the ability to directly infect and may undergo limited transmission in human populations. However, in vivo attenuation suggests additional adaptation is required for epidemic disease. Importantly, available SARS monoclonal antibodies offered success in limiting viral infection absent from available vaccine approaches. Together, the data highlight the utility of a platform to identify and prioritize prepandemic strains harbored in animal reservoirs and document the threat posed by WIV1-CoV for emergence in human populations.
Results
The discovery of SARS-like virus clusters that bridge the gap between the epidemic strains and related precursor CoV strain HKU3 virus provided the best evidence for emergence of SARS-CoV from Chinese horseshoe bats (5). Comparing the receptor binding domain (RBD), SARS-CoV Urbani and WIV1 share homology at 11 of the 14 contact residues with human ACE2 (Fig. 1A); importantly, the three amino acid changes represent relatively conservative substitution not predicted to ablate binding (Fig. 1B). Therefore, exploring WIV1 strains allows examination of emergence, pathogenesis potential, and adaptation requirements. Using the SARS-CoV infectious clone as a template (7), we designed and synthesized a full-length infectious clone of WIV1-CoV consisting of six plasmids that could be enzymatically cut, ligated together, and electroporated into cells to rescue replication competent progeny virions (Fig. S1A). In addition to the full-length clone, we also produced WIV1-CoV chimeric virus that replaced the SARS spike with the WIV1 spike within the mouse-adapted backbone (WIV1-MA15, Fig. S1B). WIV1-MA15 incorporates the original binding and entry capabilities of WIV1-CoV, but maintains the backbone changes to mouse-adapted SARS-CoV. Importantly, WIV1-MA15 does not incorporate the Y436H mutation in spike that is required for SARS-MA15 pathogenesis (8). Following electroporation into Vero cells, robust stock titers were recovered from both chimeric WIV1-MA15 and WIV1-CoV. To confirm growth kinetics and replication, Vero cells were infected with SARS-CoV Urbani, WIV1-MA15, and WIV1-CoV (Fig. 1C); the results indicate similar replication kinetics and overall titers between the CoVs. However, Western blot analysis suggests potential differences in spike cleavage/processing of WIV1 and SARS-CoV spike proteins (Fig. S1C); the ratio of full-length to cleaved spike varied between SARS spikes (Urbani, 1.21; MA15, 1.44) and WIV1 (full length, 0.61; WIV1-MA15, 0.25) signaling possible variation in host proteolytic processing (Fig. S1D). Overall, the results indicate comparable viral replication, but possible biochemical differences in processing.
Replication in Primary Human Epithelial Cells.
Next, we wanted to determine WIV-CoV replication potential in models of the human lung. Previous examination of WIV1-CoV recovered from bat samples demonstrated poor replication in A549 cells (5); however, replication of epidemic SARS-CoV is also poor in this cell type, potentially due to ACE2 expression levels (9). Therefore, well-differentiated primary human airway epithelial cell (HAE) air–liquid interface cultures were infected with WIV1-MA15, WIV1-CoV, SARS-CoV Urbani, or SARS-CoV MA15. At 24 and 48 h postinfection, both WIV1-MA15 and WIV1-CoV produce robust infection in HAE cultures equivalent to the epidemic strain and mouse-adapted strains (Fig. 1D). Together, the data demonstrate that the WIV1-CoV spike can mediate infection of human airway cultures with no significant adaptation required.
WIV1 Spike in Vivo.
To extend analysis to pathogenesis, we next evaluated in vivo infection following WIV1-MA15 and WIV1-CoV challenge. Initial studies compared WIV1-MA15 to mouse-adapted SARS-CoV (MA15) to determine spike-dependent pathogenesis. Ten-week-old BALB/c mice were infected with 104 plaque forming units (pfu) of WIV1-MA15 or SARS-CoV MA15 and followed over a 4-d time course. As expected, animals infected with SARS-CoV MA15 experienced rapid weight loss and lethality by day 4 postinfection (Fig. 2A and Fig. S2A) (10). In contrast, WIV1-MA15 induced neither lethality nor notable changes in body weight, indicating limited disease in vivo. Viral titer in the lung also revealed reduced replication following WIV1-MA15 challenge compared with control (Fig. 2B). Similarly, lung antigen staining indicated distinct attenuation of the WIV1-MA15, with most staining occurring in the airways and absent from large regions of the lungs (Fig. S2 B–D). Together, these data indicate that WIV1 spike substitution does not program pathogenesis in the mouse-adapted SARS-CoV backbone.
Discussion
The recent outbreaks of Ebola, influenza, and MERS-CoV underscore the threat posed by viruses emerging from zoonotic sources. Coupled with air travel and uneven public health infrastructures, it is critical to develop approaches to mitigate these and future outbreaks. In this paper, we outline a platform that leverages metagenomics data, synthetic genome design, transgenic mouse models, and therapeutic human antibodies to identify and treat potential prepandemic viruses. Focusing on SARS-like CoVs, the approach indicates that viruses using the WIV1-CoV spike protein are capable of infecting HAE cultures directly without further spike adaptation. Whereas in vivo data indicate attenuation relative to SARS-CoV, the augmented replication in the presence of human ACE2 in vivo suggests that the virus has significant pathogenic potential not captured by current small animal models. Importantly, therapeutic treatment with monoclonal antibodies suggests a Zmapp-based approach would be effective against a WIV1-CoV spike-mediated outbreak. However, failure of SARS DIV vaccine to induce protection highlights the need for continued development of additional therapeutics. Overall, the characterization of WIV1-CoV and its pathogenic potential highlight the utility of this platform in evaluating currently circulating zoonotic viruses.
Primary human airway epithelial cell cultures derived from human donors and grown at an air–liquid interface represent the closest model to the human lung. Therefore, the ability of both WIV1-CoV and WIV1-MA15 to grow equivalently to the epidemic SARS-CoV in these cultures is a major concern for emergence. However, pathogenesis studies in mice suggest that further adaptation may be required for epidemic disease. Compared with SARS equivalents, both full-length and chimeric WIV1 viruses had significant attenuation even with the presence of human ACE2 in the mouse model. Together, the data suggest that despite using ACE2 and robust replication in primary human airway epithelial cultures, WIV1-CoV likely maintains deficits that impact pathogenesis in mice; therefore, WIV1-mediated infection may have diminished epidemic potential in humans relative to SARS-CoV.
A number of factors may contribute to reduced mouse pathogenesis observed following WIV1-CoV spike-mediated infection. In the context of both the SARS-CoV and MERS-CoV outbreaks, focus had been primarily directed to spike binding as the key component of emergence and pandemic potential. Supported by adaption at Y436H in mouse-adapted SARS spike (10), improved binding to host receptor cannot be discounted as a crucial component in emergence. This fact is supported by improved replication of WIV1-CoV in mice expressing human ACE2 compared with control (Fig. 2D versus Fig. 3B). However, in vivo attenuation of WIV1-CoV relative to SARS-CoV Urbani despite efficient infection in primary human airway cultures suggests that additional factors contribute to epidemic emergence. One possibility is that adaptation outside of spike protein may lead to emergence via altered host–virus interactions. Whereas WIV1-MA15 was attenuated relative to SARS-MA15 in vivo, overall titers in the lung were similar to the epidemic SARS-CoV Urbani in BALB/c mice (Figs. 3 and 4). These data suggest that CoV backbone changes may account for or compensate for deficits in WIV1-CoV replication compared with SARS-CoV Urbani in vivo. Another possible factor accounting for attenuation is changes to spike that are independent of receptor binding. Whereas the receptor binding domain had garnered the most interest, changes in the remaining portion of S1 as well as the S2 portion of spike may also play a critical role in facilitating CoV infection, transmission, and/or pathogenesis (20). Differences in these regions of spike may yield increased protease targeting, enhanced spike cleavage, and/or expanded tropism leading to more robust infection for the epidemic SARS strains. Globally, the in vivo results suggest that any of these areas may have contributed to SARS-CoV emergence. However, even these interpretations must be tempered due to the robust differences between mouse and human models; further studies in nonhuman primates are required to confirm these results and derive further insight into CoV emergence from zoonotic sources.
Despite the differences in the backbone genome sequences, therapeutics developed against SARS-CoV provide some measure of protection in the context of a future outbreak. Testing the four most broadly neutralizing SARS-CoV antibodies revealed effective control of WIV1-MA15 at relatively low concentrations of antibody (14, 15, 21). For two of the four antibodies tested (Fm6 and 230.15), WIV1-CoV spike-expressing virus was neutralized equivalently or better than SARS-CoV Urbani. Similarly, only minimal differences at the low end of the neutralization curve were noted for antibody 227.14. Whereas antibody 109.8 produced only marginal neutralization of WIV1-MA15, the overall antibody neutralization data argue that multivalent monoclonal antibody approaches could limit a WIV1-CoV spike-mediated outbreak. As such, a “ZMapp”-based approach could have great potential in stemming or preventing a future SARS-CoV–like outbreak.
In contrast to the success of monoclonal antibodies, vaccine failure indicated further development and refinement are necessary. The development of a DIV SARS-CoV vaccine was buoyed as a possible means to control SARS-CoV outbreaks based on robust neutralization and protection in young mice (18). However, studies with DIV in aged animals revealed incomplete protection, significant immune pathology, and eosinophilia (19). Despite these prior results, the efficacy of monoclonal antibody treatments made further testing of DIV seemingly worthwhile against WIV1-CoV spike-mediated infection. However, the results remained the same, as vaccination of aged mice resulted in no protection from WIV1-MA15 replication in vivo (Fig. 5). Importantly, increased immune pathology and observed eosinophilia indicate that broad-based vaccination efforts against SARS-CoV–like viruses must consider heterologous viruses as well as failure due to senescence in the aged host. A number of novel platforms including Venezuelan Equine Encephalitis Virus Replicon Particle (VRP) and live-attenuated vaccine approaches show great promise in these areas, but require further testing and development before deployment in an outbreak setting (22, 23).
Overall, the results from these studies highlight the utility of a platform that leverages metagenomics findings and reverse genetics to identify prepandemic threats. For SARS-like WIV1-CoV, the data can inform surveillance programs, improve diagnostic reagents, and facilitate effective treatments to mitigate future emergence events. However, building new and chimeric reagents must be carefully weighed against potential gain-of-function (GOF) concerns. Whereas not generally expected to increase pathogenicity, studies that build reagents based on viruses from animal sources cannot exclude the possibility of increased virulence or altered immunogenicity that promote escape from current countermeasures. As such, the potential of a threat, real or perceived, may cause similar exploratory studies to be limited out of an “abundance of caution.” Importantly, the government pause on GOF studies may have already impacted the scope and direction of these studies. Whereas previous adaption of the epidemic SARS-CoV strain provided insights into species-specific changes, bat-derived WIV1 adaptation may identify elements critical for pathogenesis and transition from reservoir to human host; targets include viral proteins that interact with host machinery or host immunity like NSP1, envelope, or ORF6 (22, 24, 25). Similarly, WIV1-CoV could be used to drive improved therapeutics, including escape mutants for improved monoclonal antibodies or more broadly neutralizing vaccine approaches. However, it remains unclear from the current policies and GOF environment if these types of studies will be permissible. Although limits and standards for these types of experiments must be established, erring on the side of caution is not without its own risks and balancing the benefits of these types of studies must also be weighed against the potential hazards.
Using a novel platform to translate metagenomics findings, the WIV1-CoV cluster has been identified as a threat for future emergence in human populations due to robust replication in primary human airway epithelial cell cultures. However, based on in vivo mouse data, additional adaptations will likely be required to produce epidemic disease. Notably, whereas current antibody-based therapies hold great promise in treating WIV1-CoV spike-mediated infection, failure of SARS-CoV vaccination approaches presents a major challenge for any efforts to protect against future emergent viruses. Together, the data illustrate the utility of the platform and highlight the need to build and maintain preparations for future emergence events.
Materials and Methods
Viruses, Cells, and Infection.
Wild-type and chimeric CoVs were cultured on Vero E6 cells, grown in DMEM (Gibco) and 5% fetal clone serum (HyClone) along with anti/anti (Gibco). Growth curves in Vero and primary human airway epithelial cells were performed as previously described (26, 27). Human lungs were procured under University of North Carolina at Chapel Hill (UNC) Institutional Review Board-approved protocols.
Construction of Chimeric SARS-Like Viruses.
Both wild-type and chimeric WIV-CoV infectious clones were designed using published sequences and based on the SARS-CoV infectious clone (10). Synthetic construction of chimeric mutant and full-length WIV1-CoV were approved by the UNC Institutional Biosafety Committee and the Dual Use Research of Concern Committee.
Ethics Statement.
The study was carried out in accordance with the recommendations for care and use of animals by the Office of Laboratory Animal Welfare (OLAW), National Institutes of Health. The Institutional Animal Care and Use Committee (IACUC) of University of North Carolina (UNC permit no. A-3410-01) approved the animal study protocol (IACUC no. 13–033).
Mice and in Vivo Infection.
Female 10-wk- and 12-mo-old Balb/cAnNHsD mice ordered from the Harlan Labs were infected as previously described (23). For vaccination, young and aged mice were vaccinated and boosted by footpad injection with a 20-μL volume of either 0.2 μg of double-inactivated SARS-CoV vaccine (DIV) with alum or mock PBS as previously described (19).
Generation and Infection of ACE2 Tissue-Specific Transgenic Mice.
Transgenic mice with airway-targeted overexpression of human ACE2 were generated by microinjection of fertilized C3H × C57BL/6 (C3B6) F1 hybrid oocytes with an expression cassette consisting of the HFH4/FOXJ1 lung ciliated epithelial cell-specific promoter elements and the coding region of ACE2 cDNA in a pTG1 vector (11) (UNC Animal Model Core). Founder mice were crossed to C3B6, producing human ACE2-transgenic mice that were each previously tested for transgene expression as described in SI Materials and Methods. ACE2-transgenic mice given i.p. injection of 200 μg of human Ab S227.14 or PBS control (0.20 mL total volume, five to six mice per group) 1 d before infection as previously described (17).
Histological Analysis.
Lung tissues for histological analysis were fixed in buffered formalin phosphate 10% (4–5% wt/wt formaldehyde) (Fisher #SF100-20) for at least 7 d, tissues were embedded in paraffin, and 5-µm sections were prepared by the UNC histopathology core facility as previous described (23). Images were captured using an Olympus BX41 microscope with an Olympus DP71 camera.
Virus Neutralization Assays.
Plaque reduction neutralization titer assays were preformed with previously characterized antibodies against SARS-CoV as previously described (14, 15, 21). Briefly, neutralizing antibodies or serum were serially diluted twofold and incubated with 100 pfu of the different virus strains for 1 h at 37 °C. The virus and antibodies were then added to a six-well plate with 5 ×105 Vero E6 cells per well with n ≥ 2. After a 1-h incubation at 37 °C, cells were overlaid with 3 mL of 0.8% agarose in media. Plates were incubated for 2 d at 37 °C and then stained with neutral red for 3 h, and plaques were counted. The percentage of plaque reduction was calculated as [1 − (no. of plaques with antibody/no. of plaques without antibody)] × 100.
Statistical Analysis.
All experiments were conducted contrasting two experimental groups (either two viruses or vaccinated and unvaccinated cohorts). Therefore, significant differences in viral titer and histology scoring were determined by a two-tailed Student’s t test at individual time points. Data were normally distributed in each group being compared and had similar variance.
Biosafety and Biosecurity.
Reported studies were initiated after the University of North Carolina Institutional Biosafety Committee approved the experimental protocol: project title: Generating infectious clones of Bat SARS-like CoVs; lab safety plan ID: 20145741; schedule G ID: 12279. These studies were initiated before the US Government Deliberative Process Research Funding Pause on Selected Gain of Function Research Involving Influenza, MERS, and SARS Viruses (www.phe.gov/s3/dualuse/Documents/gain-of-function.pdf), and the current paper has been reviewed by the funding agency, the National Institutes of Health (NIH). Continuation of these studies has been requested and approved by the NIH.
Acknowledgments
We thank Dr. Zhengli-Li Shi of the Wuhan Institute of Virology for access to bat CoV sequences and plasmid of WIV1-CoV spike protein. Research was supported by the National Institute of Allergy and Infectious Disease and the National Institute of Aging of the NIH under Awards U19AI109761 and U19AI107810 (to R.S.B.), AI1085524 (to W.A.M.), and F32AI102561 and K99AG049092 (to V.D.M.). Human airway epithelial cell cultures were supported by the National Institute of Diabetes and Digestive and Kidney Disease under Award NIH DK065988 (to S.H.R.). Support for the generation of the mice expressing human ACE2 was provided by NIH Grants AI076159 and AI079521 (to A.C.S.).
Footnotes
- ↵1To whom correspondence should be addressed. Email: rbaric@email.unc.edu.
-
Author contributions: V.D.M., B.L.Y., and R.S.B. designed research; V.D.M., B.L.Y., A.C.S., S.S.A., L.E.G., T.S., J.A.P., S.R.R., J.S., and T.P.S. performed research; V.D.M., B.L.Y., R.J.P., D.C., S.H.R., A.L., and W.A.M. contributed new reagents/analytic tools; V.D.M., A.C.S., K.D., R.L.G., and R.S.B. analyzed data; and V.D.M. and R.S.B. wrote the paper.
-
The authors declare no conflict of interest.
-
This article is a PNAS Direct Submission.
-
See Commentary on page 2812.
-
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1517719113/-/DCSupplemental.
A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence
volume 21,pages1508–1513(2015 )
Abstract
The emergence of severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome (MERS)-CoV underscores the threat of cross-species transmission events leading to outbreaks in humans. Here we examine the disease potential of a SARS-like virus, SHC014-CoV, which is currently circulating in Chinese horseshoe bat populations1. Using the SARS-CoV reverse genetics system2, we generated and characterized a chimeric virus expressing the spike of bat coronavirus SHC014 in a mouse-adapted SARS-CoV backbone. The results indicate that group 2b viruses encoding the SHC014 spike in a wild-type backbone can efficiently use multiple orthologs of the SARS receptor human angiotensin converting enzyme II (ACE2), replicate efficiently in primary human airway cells and achieve in vitro titers equivalent to epidemic strains of SARS-CoV. Additionally, in vivo experiments demonstrate replication of the chimeric virus in mouse lung with notable pathogenesis. Evaluation of available SARS-based immune-therapeutic and prophylactic modalities revealed poor efficacy; both monoclonal antibody and vaccine approaches failed to neutralize and protect from infection with CoVs using the novel spike protein. On the basis of these findings, we synthetically re-derived an infectious full-length SHC014 recombinant virus and demonstrate robust viral replication both in vitro and in vivo. Our work suggests a potential risk of SARS-CoV re-emergence from viruses currently circulating in bat populations.
Methods
Viruses, cells, in vitro infection and plaque assays.
Wild-type SARS-CoV (Urbani), mouse-adapted SARS-CoV (MA15) and chimeric SARS-like CoVs were cultured on Vero E6 cells (obtained from United States Army Medical Research Institute of Infectious Diseases), grown in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, CA) and 5% fetal clone serum (FCS) (Hyclone, South Logan, UT) along with antibiotic/antimycotic (Gibco, Carlsbad, CA). DBT cells (Baric laboratory, source unknown) expressing ACE2 orthologs have been previously described for both human and civet; bat Ace2 sequence was based on that from Rhinolophus leschenaulti, and DBT cells expressing bat Ace2 were established as described previously8. Pseudotyping experiments were similar to those using an HIV-based pseudovirus, prepared as previously described10, and examined on HeLa cells (Wuhan Institute of Virology) that expressed ACE2 orthologs. HeLa cells were grown in minimal essential medium (MEM) (Gibco, CA) supplemented with 10% FCS (Gibco, CA) as previously described24. Growth curves in Vero E6, DBT, Calu-3 2B4 and primary human airway epithelial cells were performed as previously described8,25. None of the working cell line stocks were authenticated or tested for mycoplasma recently, although the original seed stocks used to create the working stocks are free from contamination. Human lungs for HAE cultures were procured under University of North Carolina at Chapel Hill Institutional Review Board–approved protocols. HAE cultures represent highly differentiated human airway epithelium containing ciliated and non-ciliated epithelial cells as well as goblet cells.SARS-CoV is a select agent. All work for these studies was performed with approved standard operating procedures (SOPs) and safety conditions for SARS-CoV, MERs-CoV and other related CoVs. Our institutional CoV BSL3 facilities have been designed to conform to the safety requirements that are recommended in the Biosafety in Microbiological and Biomedical Laboratories (BMBL), the US Department of Health and Human Services, the Public Health Service, the Centers for Disease Control (CDC) and the NIH. Laboratory safety plans were submitted to, and the facility has been approved for use by, the UNC Department of Environmental Health and Safety (EHS) and the CDC. Electronic card access is required for entry into the facility. All workers have been trained by EHS to safely use powered air purifying respirators (PAPRs), and appropriate work habits in a BSL3 facility and active medical surveillance plans are in place. Our CoV BSL3 facilities contain redundant fans, emergency power to fans and biological safety cabinets and freezers, and our facilities can accommodate SealSafe mouse racks. Materials classified as BSL3 agents consist of SARS-CoV, bat CoV precursor strains, MERS-CoV and mutants derived from these pathogens. Within the BSL3 facilities, experimentation with infectious virus is performed in a certified Class II Biosafety Cabinet (BSC). All members of the staff wear scrubs, Tyvek suits and aprons, PAPRs and shoe covers, and their hands are double-gloved. BSL3 users are subject to a medical surveillance plan monitored by the University Employee Occupational Health Clinic (UEOHC), which includes a yearly physical, annual influenza vaccination and mandatory reporting of any symptoms associated with CoV infection during periods when working in the BSL3. All BSL3 users are trained in exposure management and reporting protocols, are prepared to self-quarantine and have been trained for safe delivery to a local infectious disease management department in an emergency situation. All potential exposure events are reported and investigated by EHS and UEOHC, with reports filed to both the CDC and the NIH.
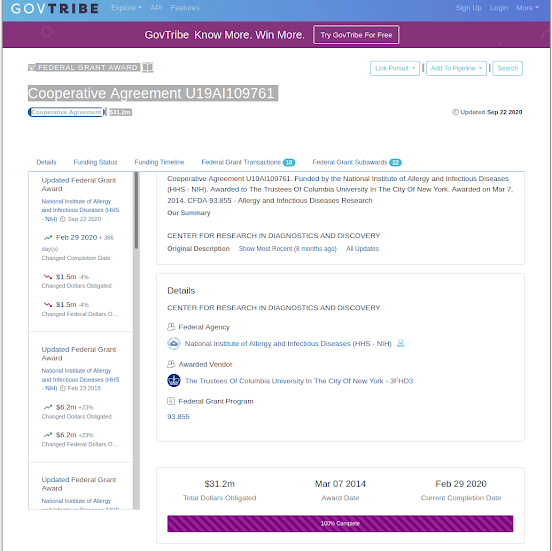
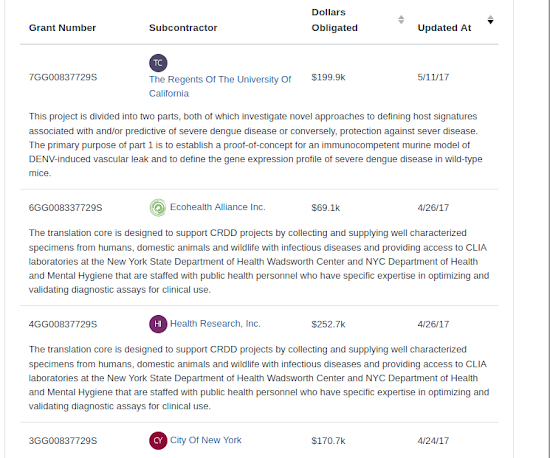
Cooperative Agreement U19AI109761
H.R. 591: Defund EcoHealth Alliance Act
Primary Source
The text of the bill below is as of Jan 28, 2021 (Introduced).
117th CONGRESS
1st Session
H. R. 591
IN THE HOUSE OF REPRESENTATIVES
January 28, 2021
Mr. Reschenthaler introduced the following bill; which was referred to the Committee on Oversight and Reform
A BILL
To prohibit Federal funding to EcoHealth Alliance, Inc., and for other purposes.
1.Short title
This Act may be cited as the Defund EcoHealth Alliance Act.
2.Prohibition on Federal Funding to EcoHealth Alliance, Inc
(a)In general
No funds authorized or appropriated by Federal law may be made available for any purpose to EcoHealth Alliance, Inc.
(b)GAO study and report
Not later than 2 years after the date of the enactment of this Act, the Comptroller General of the United States shall conduct a study, and submit to Congress a report, on the amount of Federal funds awarded to EcoHealth Alliance, Inc., during the 10-year period preceding such date of enactment were provided, whether purposely or inadvertently, to the People’s Republic of China, Chinese Communist Party, or the Wuhan Institute of Virology, or any agency or instrumentality thereof.
Alliance for Human Research Protection
Advancing Voluntary, Informed Consent to Medical InterventionWhat is Gain-of-Function Research & Who is at High Risk?
“Gain-of-function” is the euphemism for biological research aimed at increasing the virulence and lethality of pathogens and viruses. GoF research is government funded; its focus is on enhancing the pathogens’ ability to infect different species and to increase their deadly impact as airborne pathogens and viruses. Ostensibly, GoF research is conducted for biodefense purposes. These experiments, however, are extremely dangerous. Those deadly science-enhanced pathogens can, and do escape into the community where they infect and kill people. What’s more, this line of research can be used for biological warfare.
- Rumors that Iraq was preparing to use weaponized anthrax – as a “weapon of mass destruction” – provided the US government with a justification for the 2003 invasion
In 1992, Meryl Nass, MD, analyzed the characteristics of an anthrax epidemic in Zimbabwe, Rhodesia in 1978-1980, that was claimed to be a natural occurrence. Dr. Nass demonstrated that the pattern of the epidemic, the spread, and weather conditions, could not have occurred due to a natural event; it must, therefore, have been triggered as a bioweapon. She reported her findings in the journal Physicians for Social Responsibility Quarterly, 1992.[1]
Government officials and the recipients of government grants and contracts for “gain-of-function” research argue that these experiments are critical for understanding the subtle changes that can make a bird virus a pandemic threat.
Anthony Fauci, MD
Dr. Anthony Fauci, who has headed the National Institute of Allergy and Infectious Diseases (NIAID) since 1984, has played a major role in promoting and funding gain-of-function research, both in the US and China. Newsweek reported: “He argued that the research was worth the risk it entailed because it enables scientists to make preparations [ ] that could be useful if and when a pandemic occurred.”
- Those claims are belied by the empirical evidence
GoF experiments have neither prevented a pandemic, nor provided useful information about safe and effective pandemic countermeasures. Numerous prominent scientists argue that these experiments deviate from morally justifiable research, and the experimentally altered pathogens have put the entire human species at risk.
However, GoF research is defended by a closed circle of scientists within government and those who are contracted by government to conduct this line of research.
In 2011, controversy erupted when two separate teams of researchers – one headed by Ron Fouchier from the Netherlands, another headed by Yoshihiro Kawaoka from the University of Wisconsin and the University of Tokyo – announced that they had modified the H5N1 avian flu virus so that it jumped from birds to mammals and between mammals.[2],[3] Both research teams were funded by the NIH – NIAID. They used reverse genetics to build a more lethal virus by combining directed mutations and natural selection, suggesting that this H5N1 variant could be efficiently transmitted between humans.
The UK Independent reported: “An increasing number of scientists outside the influenza field have expressed concern over attempts to deliberately increase the human transmissibility of the H5N1 bird-flu virus. This is done by mutating the virus so that it can pass by airborne droplets between laboratory ferrets, the standard “animal model” of human influenza.”
Scientists, who are committed to the precautionary principle in medicine, medical research, and in public health policy, eschew GoF experimentation. These critics cite the Nuremberg Code prohibition against conducting experiments that pose a risk to human life. Such experiments “should be undertaken only if they provide humanitarian benefits that sufficiently offset the risks and if these benefits are unachievable by safer means.”
The risks posed by influenza GoF experiments include frequent documented escapes of deadly pathogens into the community, which have a potential for triggering a pandemic. These risks far outweigh any speculative benefits. What’s more, as Dr. Marc Lipsitch of Harvard and Dr. Alison Galvani of Yale argue:“the creation and manipulation of potential pandemic pathogens are too risky to justify…there are safer more effective experimental approaches that are both more scientifically informative and more straightforward to translate into improved public health.” [PLoS Medicine, 2014][4]
Dr. Marc Lipsitch, director of the Center for Communicable Disease Dynamics at Harvard School of Public Health stated that recent disease-enhancing experiments “have given us modest scientific knowledge and done almost nothing to improve our preparedness for pandemics, and yet [these experiments] risked creating an accidental pandemic.”[5]
Alison Galvani, PhD, Professor of Epidemiology (Microbial Diseases); Director of the Center for Infectious Disease Modeling and Analysis (CIDMA) of Yale University. Dr. Galvani is one of Yale’s youngest-ever tenured faculty member. She went to Oxford for her undergraduate degree and did her PhD in theoretical epidemiology with Robert May, the former head of the UK Royal Society. She received a Blavatnik Award from the New York Academy of Sciences . Dr. Galvani is an interdisciplinary scientist who prides herself on challenging dogma.
The considerable risk of laboratory enhanced transmitability of influenza viruses was obvious. Dr. Andrew Pavia, Chief, Division of Pediatric Infectious Diseases at the University of Utah stated: “A readily transmitted H5N1 virus could be extraordinarily lethal; therefore, the risk for accidental release is significant, and deliberate misuse of the data to create a biological weapon is possible.”[6]
The controversy escalated when the National Science Advisory Board for Biosecurity (NSABB) issued its recommendation (December 2011) that the controversial H5N1 reports be published with significant redactions.“Methodological and other details that could enable replication of the experiments by those who would seek to do harm” are to be redacted. The research and the NSABB recommendation polarized the scientific community which recognized that the easily transmitted H5N1 laboratory creation could be extraordinarily lethal. This laboratory-engineered virus poses a significant risk for accidental release into the community.[7]
Michael Osterholm, director of the Center for Infectious Disease Research and Policy (CIDRAP) at the University of Minnesota and a vocal critic of the decision to publish the H5N1 research, stated that the flu research community has not rigorously weighed the risks and benefits of gain-of-function studies. He stated that proponents of “gain-of-function” research have overstated the benefits, including the potential for developing better vaccines and antiviral drugs, or improving surveillance measures. “We still do H5N1 surveillance in the same way a year later.”
Adel A. F. Mahmoud, an infectious disease specialist at Princeton University and the former president of Merck Vaccines is quoted in Science: “The scientific justification presented for doing this work is very flimsy, to put it mildly, and the claims that it will lead to anything useful are lightweight… The mutations guided nothing.”
Richard Ebright, a molecular biologist at Rutgers University in Piscataway, New Jersey, cautioned that the security precautions are “insufficient and amazingly lame.” He stated in the journal Science:
“this work should never have been done”
A New York Times editorial in 2012, dubbed the experiment “An Engineered Doomsday.”
“Now scientists financed by the National Institutes of Health have shown in a laboratory how [an avian influenza virus] could kill tens or hundreds of millions of people if it escaped confinement or was stolen by terrorists. […]
The most frightening research was done by scientists at the Erasmus Medical Center in Rotterdam, who sought to discover how likely it is that the “bird flu” virus, designated A(H5N1), might mutate from a form that seldom infects or spreads among humans into a form highly transmissible by coughing or sneezing. Thus far the virus has infected close to 600 humans and killed more than half of them, a fatality rate that far exceeds the 2 percent rate in the 1918 influenza pandemic that killed as many as 100 million people.
… it looks like the research should never have been undertaken because the potential harm is so catastrophic and the potential benefits from studying the virus so speculative.”
Professor Lord May of Oxford, the former president of the Royal Society and a former chief science adviser to the UK government, is an outspoken critic about this line of research.
“The work they are doing is absolutely crazy. The whole thing is exceedingly dangerous.”
Yes, there is a danger, but it’s not arising from the viruses out there in the animals, it’s arising from the labs of grossly ambitious people.” [5]
He noted China’s poor safety track record: “The record of containment in labs like this is not reassuring. They are taking it upon themselves to create human-to-human transmission of very dangerous viruses. It’s appallingly irresponsible.”[Independent, 2013
Professor Simon Wain-Hobson, PhD, an eminent virologist at the Pasteur Institute in Paris is an outspoken critic of viral-engineering, and the risk this research poses. In a column in the journal Nature (2013), Dr. Wain-Hobson noted:
“Influenza virologists are going down a blind alley and the powers that be are blindly letting them go down that alley.”
He said it is very likely that some or all of “these hybrids could pass easily between humans and possess some or all of the highly lethal characteristics of H5N1 bird-flu.”
H5N1 GOF work — indeed all virological GOF work — should be suspended until virologists open up and engage in public discussion of their work and the issues it raises. Given that the flu community failed utterly to use the year-long hiatus to good effect, it is clear that an independent risk–benefit assessment of GOF work is needed.” Read H5N1 Viral-Engineering Dangers, Nature.pdf
Prof. Wain-Hobson stated: “The virological basis of this work is not strong. It is of no use for vaccine development and the benefit in terms of surveillance for new flu viruses is oversold.” He emphasized in Nature News the fact that this chimeric virus “grows remarkably well” in human cells: “if the virus escaped, nobody could predict the trajectory.”
As veteran investigative reporter, Sam Husseini, the communications director of the non-profit, Institute for Public Accuracy, who has closely followed this line of research, states there are probably hundreds of high containment biosafety (BSL-3 and BSL-4) laboratories. As of 2017, at least 263 laboratories were registered in the US as level BSL-3 and level BSL-4.
According to a report by the Center for Arms Control and Non-Proliferation, the probability of a flu virus release from a government laboratory into the community could become pandemic requires that “the Precautionary Principle should apply [in proceeding with this line of research]”.
Numerous pathogen escaped accidents have occurred at BSL-3 and BSL-4 labs.
The journal SCIENCE reported that multiple laboratory accidents at CDC’s highest security laboratories released smallpox vials, anthrax samples, H5N1 influenza samples, and H9N2 avian influenza pathogen. The lapses, Science reported, “at the world-renowned infectious disease research agency, are sure to raise questions about safety at other labs studying highly pathogenic agents, including university labs that are modifying influenza strains to make them more virulent.:
Former CDC director, Dr. Thomas Frieden stated:
“whatever you think about [such so-called gain-of-function studies],“I think it’s clearly the case that these incidents indicate that we need to really ensure that whatever work is done needs to be done safely and securely.”
These accidents led the government to temporarily suspend funding for gain-of-function research from 2014 to 2017 for SARS, MERS and avian flu viruses.
Husseini notes that exceptions from suspended funding included laboratories at Harvard University, the University of North Carolina, and the Wuhan Institute of Virology (WIV) laboratory, whose high risk coronavirus research was backed by Dr. Anthony Fauci, who awarded the Wuhan laboratory with a $3.7 million contract AFTER the government had suspended funding for avian flu viruses research.
When the “Ban on Making Lethal Viruses [was] Lifted,” the review process became even more secretive and opaque. Dr Francis Collins, head of the NIH, announced that there would be no public disclosure about the projects funded by the government. Critics, including microbiologist Richard Ebright of Rutgers University stated the lack of openness is “disturbing and indefensible.” He stated that clearer minimum safety standards and a mandate that the benefits ‘outweigh’ the risks were needed, rather than merely ‘justifying’ them.”
The Washington Post reported that in January 2018, urgent cables from the US Embassy warned that the Wuhan laboratory’s operations had serious safety problems. One of the cables specifically warned that the lab’s work on bat coronaviruses and their potential human transmission represented a risk of a new SARS-like pandemic.[8]
Dr. Fauci appears to have ignored those cables and the Wuhan Institute’s requests for assistance. Despite alarms raised about lax safety at the Wuhan laboratory, Dr. Fauci provided an additional $3.7 million grant in 2019 for “gain-of-function research for the purpose of understanding how bat coronaviruses could mutate to attack humans”
Read: Is COVID-19 the Result of a US Government- Funded Experiment in China?
Newsweek reported that this phase of the research at the Wuhan laboratory in 2019, was run by Peter Daszak, of EcoHealth Alliance, a so-called nonprofit that has received millions of dollars from the US government during the Obama and Trump administration .
On May 12th, The Washington Post reported that EcoHealth is a “longtime partner” of the Wuhan Institute of Virology. Given the Post’s earlier report about the US Embassy cables warning about major safety hazards at the Wuhan lab, and in light of the Chinese government refusal to allow an independent investigation into the origins of the global pandemic, the Washington Post acknowledged:
“Theoretically, an accident during such activities could prompt an outbreak. If researchers, for example, had found SARS-CoV-2, the virus that causes covid-19, and cultured the virus in the lab, it could have infected humans as the result of a mishap.”
Daszak, who has a multi-million dollar stake in Chinese bat research, dismissed the possibility outright: “I have never heard anything suspicious from this lab. It’s a preposterous idea”.
Honest scientists recognize and warn about the danger posed by GoF research and the potential for unleashing a lethal pandemic. Documented indisputable evidence confirms continued safety hazards at the high security laboratories from which pathogens regularly escaped into the community.
A report by GM Watch (May 20th) focuses on the hazardous bat and coronavirus experiments at the Wuhan laboratory. Dr Richard Ebright alerted the public about evidence that the Wuhan Institute and US-based researchers were genetically engineering bat viruses to investigate their ability to infect humans. He stated that they experimented with commonly used methods that leave no sign or signature of human manipulation. He cited a scientific paper published in PLoS (2017) by Wuhan scientists, including Shi Zhengli, the virologist leading the research into bat coronaviruses, who worked in collaboration with Peter Daszak.
that funding for the experiment was shared between Chinese and US institutions, including the National Institutes of Health and US Agency for International Development.
“The researchers report having conducted virus infectivity experiments where genetic material is combined from different varieties of SARS-related coronaviruses to form novel “chimeric” versions. This formed part of their research into what mutations were needed to allow certain bat coronaviruses to bind to the human ACE2 receptor – a key step in the human infectivity of SARS-CoV-2.
The WIV scientists did this, Ebright points out, “using ‘seamless ligation’ procedures that leave no signatures of human manipulation”. This is noteworthy because it is a type of genetic engineering that Andersen and his team excluded from their investigation into whether SARS-CoV-2 could have been engineered – and it was in use at the very lab that is the prime suspect for a lab escape.
The evidence rebuts claims by journalists and some scientists that the SARS-CoV-2 virus responsible for the current COVID-19 pandemic could not have been genetically engineered because it lacks the “signs” or “signatures” that supposedly would be left behind by genetic engineering techniques.”
Dr. Ebright cited a just-published pre-print scientific report by the Wuhan scientists who describe how they “spiked proteins from bat SARS-related CoV (SARSr-CoV), among other coronaviruses, to bind to bat and human ACE2 receptors – in other words, how efficiently they infect humans.” He points out that the paper states, “All work with the infectious virus was performed under biosafety level 2 conditions” which is not suitable for such high risk experimentation. This level is suitable for work involving agents of only “moderate potential hazard to personnel and the environment”.
Dr Jonathan Latham, a virologist, is Executive Director of the Bioscience Resource Project, which conducts independent scientific analysis of genetic engineering and its risks. He is the Editor of Independent Science News. He criticised the research on bat coronaviruses that has been taking place in Wuhan and the US. He argues that these experiments are “providing an evolutionary opportunity” for such viruses “to jump into humans.” He emphasized that the experiments were “providing opportunities for contamination events and leakages from labs, which happen on a routine basis”.
GM Watch notes: “Given that lab accidents are common, including in China where the SARS virus has escaped from high-level containment facilities multiple times, the details emerging about the research activities of the WIV and US scientists again underline the need for a credible independent investigation of the most forensic kind into the origins of the current pandemic. And a broader investigation is also needed into the full range of biological threats arising from various areas of potentially hazardous but laxly regulated biotechnology research.”
Inside America’s Secretive Biolabs,
an investigative report by USA Today May 2015 reveals hundreds of accidents, safety violations and near misses put people at risk.
“Vials of bioterror bacteria have gone missing. Lab mice infected with deadly viruses have escaped, and wild rodents have been found making nests with research waste. Cattle infected in a university’s vaccine experiments were repeatedly sent to slaughter and their meat sold for human consumption. Gear meant to protect lab workers from lethal viruses such as Ebola and bird flu has failed, repeatedly.
A team of reporters who work for the USA TODAY Network of Gannett newspapers and TV stations identified more than 200 of these high-containment lab facilities in all 50 states and the District of Columbia operated by government agencies, universities and private companies. They’re scattered across the country from the heart of New York City to a valley in Montana; from an area near Seattle’s Space Needle to just a few blocks from Kansas City’s Country Club Plaza restaurant and shopping district.”
Human Error in High-Biocontainment Labs: A Likely Pandemic Threat, by Lynn Klotz, Bulletin of the Atomic Scientists, 2019
“Incidents causing potential exposures to pathogens occur frequently in the high security laboratories often known by their acronyms, BSL3 (Biosafety Level 3) and BSL4. Lab incidents that lead to undetected or unreported laboratory-acquired infections can lead to the release of a disease into the community outside the lab; lab workers with such infections will leave work carrying the pathogen with them. If the agent involved were a potential pandemic pathogen, such a community release could lead to a worldwide pandemic with many fatalities. Of greatest concern is a release of a lab-created, mammalian-airborne-transmissible, highly pathogenic avian influenza virus, such as the airborne-transmissible H5N1 viruses created in the laboratories of Ron Fouchier in the Netherlands and Yoshihiro Kawaoka In Madison Wisconsin.
Such releases are fairly likely over time, as there are at least 14 labs (mostly in Asia) now carrying out this research. Whatever release probability the world is gambling with, it is clearly far too high a risk to human lives. Mammal-transmissible bird flu research poses a real danger of a worldwide pandemic that could kill human beings on a vast scale.”
Dr. Fauci, the head of the NIAID since 1984, has been in the forefront in supporting highest risk pathogen experiments. Dr. Fauci bears grave responsibility for having ignored a continuous series of documented reports — all of which warned of impending catastrophic pandemics, directly caused by experimental. laboratory-created pathogens.
It should be evident to everyone, that as long as irresponsible government officials continue to fund and promote experiments whose aim is to increase the virulence and lethal capacity of biological pathogens and viruses, the risk that those lethal pathogens can, have, and will escape from laboratories, is certain.
- Those accidental escapes pose catastrophic existential risk for the global human community.
- If we want to preserve our existence on the planet, our government must stop funding this line of research.
Inside America's secretive biolabs
Investigation reveals hundreds of accidents, safety violations and near misses put people at risk
https://archive.is/RY3heSummary
This article contains the records of researcher Shi Zhengli of Wuhan Institute of Virology, Chinese Academy of Sciences published on the coronavirus, and discusses the possible source of the Covid-19 virus discovered in December 2019 from a research perspective. Shi Zhengli's research group and others in Mainland China/Hong Kong The research status of the research team and the virus research status of the Covid-19 virus in the worldwide outbreak. This article does not take any established position to ensure the objectivity of the information.
Short link to this article: https://bit.ly/2ydLPCc
Shi Zhengli's personal information and research experience
Researcher Shi Zhengli was born in Xixia County, Henan Province, China in May 1964. She graduated from Wuhan University, China in 1987, received a master's degree from the Wuhan Institute of Virology, Chinese Academy of Sciences (CAS) in 1990, and from Montpellier, France in 2000. Obtained a Ph.D. from Montpellier 2 University in France (Ph.D. from Montpellier 2 University in France).
After the SARS incident broke out in 2003, Shi Zhengli personally led a research team to investigate bat habitat caves across the country, collect samples of various bats for virus testing, and look for traces of SARS virus. In 2005, the research team led by Shi Zhengli and Cui Jie found that the SARS virus originated in bats (the SARS virus originated in bats), and the results were published in Science in 2005 and Journal of General Virology in 2006.
Since 2014, Shi Zhengli has been a grantee of some US government funded projects that have funded coronavirus research and some of the National Basic Programs of China, the Chinese Academy of Sciences, the National Natural Science Foundation of China, and the Strategic Limited Program of the Chinese Academy of Sciences.
In 2014, Shi Zhengli participated in a joint study of bat coronaviruses between the University of North Carolina and Wuhan Institute of Virology, with Ralph S. Baric as the main researcher. In the same year, the US government announced that it would suspend research on dangerous virology involving influenza, MERS & SARS viruses (influenza, MERS & SARS viruses), and funding for this project was suspended.
In 2015, Shi Zhengli participated in research on coronaviruses that can infect humans . In November 2015, researchers from the University of North Carolina at Chapel Hill published a paper on the synthesis of a SARS-like coronavirus in Nature Medicine. Shi Zhengli is also one of the authors .
In August 2017, Shi Zhengli published a paper suggesting that the source of the SARS virus came from the Chinese chrysanthemum bat in the bat cave in Yunnan .
In April 2018, Shi Zhengli’s team published a paper in Nature, claiming that they discovered a new type of coronavirus that caused the deaths of large-scale swine acute diarrhea in Guangdong, and named it Porcine Acute Diarrhea Syndrome Coronavirus (SADS Coronavirus) . The genome sequence of this virus is as high as 98% with some viruses isolated from samples of Guangdong chrysanthemum head bat .
During the 2019-2020 coronavirus pandemic, Shi Zhengli and other scientists from the Institute formed a new coronavirus research (Covid-19; SARS-Cov-2) expert group. In February 2020, researchers led by Shi Zhengli published a titled "A pneumonia outbreak associated with a new coronavirus that may have originated from bats" in the journal Nature. new coronavirus of probable bat origin”), it is said that the new coronavirus (SARS-CoV-2) is the same family as SARS, and is the closest to a coronavirus found in bats. In February 2020, her research group published an article in Cell Research, demonstrating that an experimental drug owned by Gilead Sciences, remdesivir (remdesivir), In inhibiting the virus in vitro (in inhibiting the virus in vitro) has a positive effect, and represented the Wuhan Institute of Virology to apply for a patent for the application of this drug in China. Shi Zhengli wrote a paper as a co-author, marking the virus as the first Disease X.
In February 2020, the South China Morning Post reported that Shi Zhengli had built the world's largest bat-related virus database in 10 years, which provided help for the scientific community to understand the virus. The South China Morning Post also reported that Shi Zhengli became the focus of personal attacks on Chinese social media. They said that the Wuhan Institute of Virology was the source of the virus, leading Shi Zhengli to post: "I swear by my life that the virus has nothing to do with the laboratory." When asked about comments on the attack, Shi Zhengli replied: "My time must be spent on more important things." Caixin reported that Shi Zhengli made a further public statement on the source of the new crown virus. She said: "New crown virus It is used by nature to punish humans for maintaining uncivilized living habits. I, Shi Zhengli, swear by life, it has nothing to do with our laboratory.
Covid-19 virus research timeline
- The British-German team traced the source of the new "gene archaeology" to find the ancestor genome coronavirus through (2020-04-10)
Shi Zhengli's papers and media records
-
Li, Wendong; Shi, Zhengli; Yu, Meng; Ren, Wuze; Smith, Craig; Epstein, Jonathan H; Wang, Hanzhong; Crameri, Gary; Hu, Zhihong; Zhang, Huajun; Zhang, Jianhong; McEachern, Jennifer; Field , Hume; Daszak, Peter; Eaton, Bryan T; Zhang, Shuyi; Wang, Lin-Fa (28 Oct 2005). "Bats Are Natural Reservoirs of SARS-Like Coronaviruses". Science. 310 (5748): 676-679. Bibcode : 2005Sci...310..676L . Doi : 10.1126/ science.1118391 . PMID 16195424 .
-
Lu Wei (鲁伟); Liu Zheng (刘铮) (10 March 2009). "Archived copy" Shi Zhengli: a female scientist with a virus . sciencenet.cn (in Chinese). http://news.sciencenet.cn/sbhtmlnews /2009/3/216816.htm l" rel="nofollow">Archived from the original on 7 February 2019. Retrieved 26 January 2020.
-
Ren, Wuze; Li, Wendong; Yu, Meng; Hao, Pei; Zhang, Yuan; Zhou, Peng; Zhang, Shuyi; Zhao, Guoping; Zhong, Yang; Wang, Shengyue; Wang, Lin-Fa; Shi, Zhengli ( 1 November 2006). "Full-length genome sequences of two SARS-like coronaviruses in horseshoe bats and genetic variation analysis". J Gen Virol. 87(11): 3355–3359. doi : 10.1099/vir.0.82220-0 . PMID 17030870 .
-
Ge, X., Li, J., Yang, X. et al. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature 503, 535–538 (2013). https://doi.org/10.1038/nature12711
-
Menachery, Vineet D.; Yount, Boyd L.; Debbink, Kari; Agnihothram, Sudhakar; Gralinski, Lisa E.; Plante, Jessica A.; Graham, Rachel L.; Scobey, Trevor; Ge, Xing-Yi. A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence . Nature Medicine. 2015-12, 21 (12): 1508–1513.
-
Jiazheng Xie, Yang Li,Xurui Shen, Geraldine Goh, Yan Zhu. Jie Cui, Lin-Fa Wang, Zheng-Li Shi, PengZhou, Dampened STING-Dependent Interferon Activation in Bats, Cell Host and Microbe, Volume 23, Issue 3, 14 March 2018, Pages 297-301.e4
- [Yangtze River Daily] Wuhan scientists led the research and found that the "culprit" of piglet diarrhea was bats . http://www.whiov.ac.cn. [2020-02-15] . (Original content http://www.whiov.ac .cn/xwdt_105286/kydt/201804/t20180428_5004442.htm l" rel="nofollow">Archived on 2020-02-15).
-
Zhang, Wei; Du, Rong-Hui; Li, Bei; Zheng, Xiao-Shuang; Yang, Xing-Lou; Hu, Ben; Wang, Yan-Yi; Xiao, Geng-Fu; Yan, Bing; Shi, Zheng- Li; Zhou, Peng (1 January 2020). "Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes" . Emerging Microbes & Infections. 9 (1): 386–389. doi : 10.1080/22221751.2020. 1,729,071 . PMC 7,048,229 . PMID 32,065,057 .
-
Zhang Jiawei. Research found that a new type of porcine coronavirus originated from bats (news report). Xinhua News Agency. April 5, 2018 [February 6, 2020]. (Original content http://www.xinhuanet.com/tech /2018-04/05/c_1122641615.ht m" rel="nofollow">Archived on February 22, 2020) (Chinese).
-
Zhou, P., Yang, X., Wang, X. et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273 (2020). https://doi.org/10.1038/s41586-020-2012-7
-
-
-
This article contains the records of researcher Shi Zhengli of Wuhan Institute of Virology, Chinese Academy of Sciences published on the coronavirus, and discusses the possible source of the Covid-19 virus discovered in December 2019 from a research perspective. Shi Zhengli's research group and others in Mainland China/Hong Kong The research status of the research team and the virus research status of the Covid-19 virus in the worldwide outbreak. This article does not take any established position to ensure the objectivity of the information
-
12 monkeys 👇 actual 12 monkeys 👇
12 monkeys 👇 actual 12 monkeys 👇
- The Virus Cancer Program
1964-1980 - The Virus Cancer Program had it roots in 1964 when Congress provided funds to the National Institutes of Health (NIH) for intensive research into the possible role of viruses in leukemia. In 1968 the Program, then titled the Special Virus-Cancer Program, was enlarged to encompass all types of cancer. On July 1, 1973 the Special Virus Cancer Program was renamed The Virus-Cancer Program (VCP) "to integrate the Program's research activities into the framework of the new National Cancer Plan."
- The Program combined the talents of many of the nation's finest virologists, biochemists, immunologists, molecular biologists, epidemiologists, and physicians, in an attempt to uncover the viral cause of cancer. Two classes of cancer-causing viruses were studied extensively: the RNA-type tumor "retroviruses" (like HIV) and the DNA herpes-type viruses (like the KS virus).
- The main goals were to collect various forms of cancer tissue and test them in animals; to identify animal and human cancer-causing viruses; to grow large amounts of "candidate human viruses" for testing purposes; and to develop vaccines against these cancer viruses. In essence, the scientists wanted to learn how to use viruses to make cancer - and to force "normal" cells to become cancerous by subjecting to viruses.
- I have studied the annual Virus Cancer Reports (VCP) covering the years 1971-1974 and 1976-1978. Each report is 300-400 pages, and the cumulative volumes refer to thousands of animal cancer virus and genetic engineering experiments.
- Biological warfare research, monkey research, and the VCP
- The annual VCP Reports must be studied with an awareness that the Program became wedded to secret military biological warfare research in the early 1970s.
- On October 18, 1971, as part of Richard Nixon's War on Cancer, the army's biowarfare research laboratory at Fort Detrick, Maryland, was permanently joined with the National Cancer Institute; and was re-titled . the Frederick Cancer Research Center. Litton Bionetics was named as the military's prime contractor.
- The primary task of the new Center was "the large scale production of oncogenic (cancer-causing) viruses and suspected oncogenic viruses to meet research needs on a continuing basis." Special attention was given to primate viruses (the alleged African source of HIV and the new KS virus)- and to the successful propagation of significant amounts of "human candidate viruses." Candidate viruses were defined as animal or human viruses that might cause human cancers. Later, the objective was to determine if such viruses could induce (either alone or with other co-carcinogens) human cancers (1977;58). Biowarfare scientists also had a keen interest in the role of human and non-human primate viruses as "helper viruses" in the production of cancer (1978;54).
- A steady supply of research animals (monkeys, chimpanzees, mice, cats, etc. ) was necessary; and multiple breeding colonies were established for the VCP. For example, a total of 2,274 primates from Africa and Asia were shipped to Litton for military use in 1971.
- Forcing cancer viruses into primates and other animals
- To induce primates and other research animals to acquire cancer,
- their immune system was deliberately suppressed by drugs, radiation, or
- cancer-causing chemicals or substances. The thymus gland and/or the spleen
- were removed, and cancer tissue and cancer viruses were injected into newborn animals or into the womb of pregnant animals. Some animals were deliberately infected with malaria to keep them chronically sick and immunodepressed.
- The U.S. is the world's leading consumer of primates, and 55,000 are used yearly in medical research. Primates (especially newborn and baby chimpanzees) are the most favored lab animals because they are most similar biochemically and immunologically to human beings. Humans share 98.4% of their DNA with chimpanzees. Chimps were extensively used by the VCP because there would be no official testing of cancer viruses on humans.
- Robert Gallo, the discoverer of HIV in 1984, was a project officer of a primate study contracted by Litton Bionetics that pumped cancerous human tissue, as well as a variety of primate and other viruses, into newborn macaques (a small species of monkey used as an animal model for human cancer).
- The actual number and identity of all the primate viruses created and adapted to human tissue during the 14 years of the SVCP is not known. In addition, some primates were released back into the wild carrying lab viruses with them. This fact is always ignored by molecular biologists searching for "viral ancestors" in the African bush,
- By the early 1970s, experimenters had transferred cancer-causing viruses into several species of monkeys. Herpesvirus saimiri, a monkey virus discovered in 1967 in the squirrel monkey, has a close genetic relationship to the new KS herpes virus. H. saimiri virus is harmless in the squirrel monkey, but when the virus was forced in the lab to "jump species" into different animal species, such as the owl monkey, marmosets and rabbits, it produces cancer in the form of fatal malignant lymphoma.
- By 1971 Dharam V Ablashi of the NCI succeeded in transferring H. saimiri, into various cell lines of human origin. (1971;35). Cancer-causing cat and hamster viruses were also engineered into macaques and other monkey species.
- By the early 1970s, it was recognized that forms of human leukemia and lymphoma were associated with herpes-type viruses. Herpes saimiri, a DNA-type virus, became the experimental model for the study of human leukemia and lymphoma. "Thus far, the only DNA viruses associated with natural cancer of animals and man are herpes viruses" (1973;15).
- Luis Melendez of Harvard studied additional primate herpes viruses (H. ateles, H. aotus, and H. saguinus) and determined their ability to induce cancer (1973;247). Attempts were made "to find a suitable method for the large-scale production of high-titer Herpesvirus saimiri" (1973;264). Researchers knew: "The clinical and immunological picture of human lymphoma and leukemia is closely approximated by the malignant disease induced in susceptible non-human primates by H. saimiri." (1973;265).
- By 1976 it was also learned that H.saimiri could spread by "contact transmission" between squirrel and owl monkeys in the laboratory.
- A monkey virus injected into humans via polio vaccines in the 1950s
- There are inherent dangers in vaccine production. Many vaccines are made on living cells; and accidental contamination with bacteria, mycoplasma, viruses, and newly-recognized "nanobacteria" are constant problems during the manufacturing process. Laboratory additives (such as fetal bovine [cow] serum) may also be a source of contamination. Half the flu vaccine supply for 2004 had to be destroyed due to contamination with disease-causing bacteria.
- Some researchers believe that injecting living and killed viruses into the body can result in these viruses combining with other viruses normally present in the body, resulting in the formation of new viral disease-causing "recombinants." The dangers of vaccines are downplayed to assure the public that vaccines are safe.
- The possibility that cancer-causing primate viruses could have been "introduced" into gays, via the experimental hepatitis B vaccine, cannot be dismissed as paranoid fantasy. In this regard, we are told that HIV is the first primate virus to "jump species" to produce an epidemic in millions of humans. But, in truth, the AIDS epidemic is the second instance in which a monkey virus has been transferred to humans.
- A cancer-causing monkey virus called "simian virus 40" (SV40) jumped species a half century ago when virus-contaminated polio vaccines were injected into millions of people, including half the U.S. population of that era. (For details, see: www.sv40cancer.com) Government health officials insist there is no proof that SV40 causes human cancer. However, independent research over the past decade indicates SV40 is clearly associated with rapidly-fatal cancers of the lung (mesothelioma), bone marrow cancer (multiple myeloma), brain tumors in children, and other forms of cancer.
- A Washington Times report (September 21, 2003) states, "Some of the polio vaccine given to millions of American children from 1962 until 2000 could have been contaminated with a monkey virus that shows up in some cancers, according to documents and testimony to be delivered to a House committee Wednesday." The SV40 story is detailed in the recently published, The Virus and the Vaccine: The True Story of a Cancer-Causing Monkey Virus, Contaminated Polio Vaccine, and the Millions of Americans Exposed.
About Litton Bionetics Inc:
[listed in virus cancer 1967 & 71 👆 as Bionetics labs]
Litton Bionetics - Applied Science Division - Frederick Cancer Research and Development Center is located at 0 Fort Detrick in Frederick, MD - Frederick County and is a business listed in the categories Research Services, Foundations Educational Philanthropic Research, Medical Research Facilities, Research And Development In The Physical, Engineering, And Life Sciences, Research And Development In The Social Sciences And Humanities, Commercial Physical & Biological Research, Commercial Economic, Sociological & Educational Research, Research & Development Centers and Research & Development Services.
Upon returning to Cleveland after taking the injection of Tetrasil, Dr. Graves had tests done at the Cleveland Veterans Administration Hospital. Those test results showed that after two weeks his T cell count had increased by 74%, and that his blood pressure rate was that of a 25 year old.
Two months later the improvement in his physical health, in general, as well as his relief from years of constant pain, extremely low vitality and energy level continues. He's now doing a little jogging, playing a bit of tennis and doing one armed push ups.
Dr Boyd E. Graves, J.D.,is the leading researcher into the origin of AIDS/HIV, legal advocate for the people and author : The Evidence of the Laboratory Birth of AIDS(San Diego, CA) – Human Rights activist and HIV/AIDS advocate American lawyer Dr. Boyd Ed Graves died Thursday at the University of California San Diego Medical Center. Dr. Graves was 57.
Dr. Graves' two decades of human rights' work, judicial activism and research on behalf of people living with HIV/AIDS, catapulted him into the world spotlight earning him both international acclaim for his bravery and dedication as well as criticisms for his controversial conclusions about the man-made origins and purpose of the HIV/AIDS virus.
Known by “Ed” to his friends and family, Dr. Graves was a dynamic and patriotic individual who dedicated his professional and personal life to the disabled, disenfranchised and the fair daily existence of men and women worldwide.
Graves began demonstrating leadership qualities at a young age and after graduating Youngstown High School with honors as Senior Class President at age 17; Graves was recommended by his Congressional Representative for an appointment at the U.S. Naval Academy in Annapolis, Maryland. Graves was one of only 30 African Americans in the country to earn the honor. During his time at U.S. Naval Academy, Graves learned Mandarin Chinese and was a member of the Navy boxing team. Graves became the first African American elected as President of the U.S. Navy Glee Club and was the first African American featured in U.S. Navy recruitment commercials. At a time of extreme racial tensions and as one of the few black plebes, Graves received many hazing, harassments, and racial slurs inside the ranks. His experiences during this time later were featured in several books and articles authored by official U.S. Navy historian Robert Schneller.
Having successfully served President Richard Nixon's administration posted aboard the USS Buchanan as a Communications Officer, Graves left military life with honorable discharge and began his civilian career entering the work force for IBM in 1977.
Civilian work place echoed his military experience, and Graves again faced with discrimination took the higher ground. In 1987, he successfully pursued a discrimination lawsuit and his appeals to the United States Supreme Court. This was the first of several case appearances in the U.S. Supreme Court that Dr. Graves would make to deter discrimination for himself and others.
In 1992 Graves graduated as a lawyer with honors earning his Juris Doctorate from Ohio Northern University School of Law in Ada Ohio and during a routine physical exam several weeks before graduating law school, Graves tested HIV positive.
With his new diagnosis, law degree and experiences as a minority, Graves went to work as a legal consultant specializing in code compliance and enforcement for the Americans with Disabilities Act to ensure people with disabilities had equal access.
Determined to understand his new diagnosis with a 'new' disease, Graves immediately began researching HIV/AIDS intent on understanding how to best preserve his health and help himself survive. In 1993 Graves' research into HIV/AIDS led him to a formerly secret federal virus development initiative coordinated by the Pentagon called, “The United States Special Virus Program.”
“The United States Special Virus Program.” involved the complicity of two government agencies, the Pentagon as coordinator seeking biowarfare solutions and the National Institute of Health as administrator. The veneer goal of the NIH program was to meet Nixon’s challenge of a war on cancer. Unfortunately for the program participants, the goal came at a cost of using humans / in vivo subjects to produce viruses, deadly viruses including the HIV/AIDs virus. For 16 years, between 1962 to 1978, the National Institute of Health, published 15 annual progress reports. Each year in this history, a report details thousands of human and non-human virus experiments seeking 'candidate viruses'. The most concise salient microcosm of this Mengele-madness was the 1971 U.S. Special Virus Research Logic Flow Chart. The secret blueprint coordinates every experiment and contract inside the U.S. Special Virus Program and demonstrates the true intent of the secret research – live human subjects, US citizens, US veterans, your brothers, uncles, cousins, friends, and neighbors.
On September 28, 1998 Dr. Graves filed his first class action lawsuit in the Ohio Federal Court in forma pauperis, seeking immediate investigation into the formerly secret U.S. Special Virus Program, including a petition for make whole relief for the class of members infected by HIV/AIDS.
On January 1, 2000 The United States Department of Justice notified Graves, they had named The Office of the President of the United States as the primary defendant in the case. On election day November 7, 2000 the sixth circuit federal court silently dismissed the case, Graves vs The President of the United States. Graves appealed and on April 11, 2001 appeared in the United States Supreme Court. The court quietly dismissed the case without comment and instructions 'not to publish.' Determined and armed with the 1971 Flow Chart, 15 years of 'missing medical history' and the evidence of the laboratory birth of AIDS, Graves continued filing litigation requesting immediate investigation into the formerly secret tax payer funded program until his death.
In 2001 Dr. Graves became the first American and African American to receive an injection of Tetrasil, the U.S. Patented Cure for AIDS (Patent # 5676977). Almost immediately, Graves health began recovering from years of damage inflicted by the 'special HIV virus' and he became an outspoken proponent of the Tetrasil treatment demanding immediate clinical trials and world wide accessibility for people living with HIV and dying of AIDS. Soon afterward Tetrasil was recalled by the patent owner/manufacturer, Dr. Marvin Antleman and Antleman Technologies, Inc. without public explanation. Graves took his experiences and requests to the Congress, General Accounting Office, the Centers for Disease Control, United Nations, World Health Organization, and several Ministers of Health around the world with varying degrees of success including China, the UK, and several African countries where he was widely revered and respected as the 'Man Who Solved AIDS'.
-
Dr Fauci DR R Gallo Worked @ Fort Frederick on this program</pubmed.ncbi.nlm.nih.govAIntramural Research Support Program, SAIC Frederick,Division of Basic Sciences, National Cancer Institute-FrederickCancer Research and Development Center,Frederick Maryland21702-1201, USA.Mikovits @ fcrfv1. ncifcrf .gov [ Judys NCI gov email }
 Infection with human immunodeficiency virus type 1 upregulates DNA methyltransferase, resulting in...The immune response to pathogens is regulated by a delicate balance of cytokines. The dysregulation of cytokine gene expression, including interleukin-12, tumor necrosis factor alpha, and gamma...united_states_special_virus_program_progress_report_4>https://www.mediafire.com/file/5h4a500o4ujkm65/united_states_special_virus_program_progress_report_4_1967_NIH_NCI_US/file</a</divnbsp</divus-special-virus-program-progress-report-8-1971.pdf
Infection with human immunodeficiency virus type 1 upregulates DNA methyltransferase, resulting in...The immune response to pathogens is regulated by a delicate balance of cytokines. The dysregulation of cytokine gene expression, including interleukin-12, tumor necrosis factor alpha, and gamma...united_states_special_virus_program_progress_report_4>https://www.mediafire.com/file/5h4a500o4ujkm65/united_states_special_virus_program_progress_report_4_1967_NIH_NCI_US/file</a</divnbsp</divus-special-virus-program-progress-report-8-1971.pdfDr Judy Mikovits | Former AIDS Scientist
Cure for AIDS may have been on downed flight MH17, says expert
Scientific community reels at reports that HIV researchers and activists headed for AIDS conference in Australia were on Malaysia Airlines flight shot-down over Ukraine.
A number of the world’s top researchers and advocates were on board Malaysia Airlines flight MH17 when it was shot down in a missile attack over Ukraine killing all 298 passengers and crew, including 28 Australians.
It is understood a number of delegates to the 20th International AIDS 2014 conference, due to start in Melbourne, Australia on Sunday were booked on the doomed jet.
The flight from Amsterdam was bound for Kuala Lumpur, a major transit hub for onward flights to Australia.
It is as yet unconfirmed exactly how many conference attendees were amongst the plane’s passengers. However, delegates to a plenary session held in Sydney ahead of the Aids 2014 event were told on Friday that email exchanges showed about 100 of their fellow attendees were travelling on the downed aircraft.
Trevor Stratton, a Canadian HIV researcher in Australia ahead of the conference told the ABC it was a massive loss to the scientific community.“What if the cure for AIDS was on that plane? Really? We don’t know”, he said
“There were some really prominent researchers that have been doing this for a very long time and we’re getting close to vaccines and people are talking about cures and the end of AIDS.
“And you can’t help but wonder what kind of expertise was on that plane.”
Prof. Richard Boyd, director of the Monash Immunology and Stem Cell Laboratories, told Guardian Australia: “There were some serious HIV leaders on that plane.”
“This will have ramifications globally because whenever you lose a leader in any field, it has an impact. That knowledge is irreplaceable,” he said.
“We’ve lost global leaders and also some bright young people who were coming through. It’s a gut-wrenching loss. I was involved in the aftermath of 9/11 in New York and it brings back that level of catastrophe.
“But the Aids community is very close-knit, like a family. They will unite and this will galvanise people to strive harder to find a breakthrough. Let’s hope that, out of this madness, there will be new hope for the world.”
Clive Aspin, a veteran HIV researcher who was at the plenary session, said the loss was “devastating“.
“There’s a huge feeling of sadness here, people are in floods of tears in the corridors.
“These people were the best and the brightest, the ones who had dedicated their whole careers to fighting this terrible virus.”
IMAGE: Signage for the 20th International AIDS Conference on July 18, 2014 in Melbourne, Australia. Twenty-eight Australians and a *number of AIDS activists, researchers, health workers and delegates bound for the major conference were among those aboard Malaysia Airlines Flight MH17 which was reportedly shot down over Eastern Ukraine.Did we loss all knowledge for the cure of aids on flight MH17 Malaysian airlines
Its to be feared that we have lost all knowledge for the cure of Aids. Together with top scientists that were on board of flight MH17 . about hundred people were on there way to this Aids conference in Melbourne. When the Boeing 777 was shot down. One of the top scientist Joep Lange and CEO of the international Aids society who worked on the university of Amsterdam . He was involved in finding for a cure sinds the disease came known in the eighties .He researched anti retro therapies that lets aid be controllable in daily life.
As many as 100 of the world’s leading HIV/Aids researchers and advocates may have been on the Malaysia Airlines flight that crashed in Ukraine, in what has been described as a “devastating” blow to efforts to tackle the virus. Delegates to a plenary session held ahead of the Aids 2014 conference were told that email exchanges showed about 100 attendees were booked on the MH17 flight. The plane was downed in eastern Ukraine by what the US and Australian governments have described as a surface-to-air missile. There was no official confirmation of the number of researchers on board
Are we facing another conspiracy that this was another way to eliminate scientific breakthrough of curing Aids?
What if the cure for Aids was on board of that plane? Then now not only 289 poor souls have lost their lives but also people suffering aids will be the victim of this stupidity.
December 1st is World AIDS Day
Yep, and it’s an annual effort held all around the globe to commemorate those who have died from the disease while giving solitary support to those who are living with it. Now, growing up we’ve always had these pertandingan melukis and all that, exposing us Malaysian kids to this the danger of AIDS and HIV and how it kills.
But as we move on into different phases in life, we forget about the disease. Unless someone in particular in our lives has the disease, then chances are we’d probably know about it a little more. But for the rest of us, the knowledge about AIDS is like a tiny chunk of meat at the back of our brains – we know it’s there, but we don’t know much about it. So fast forward to today… What DO we know about the disease in Malaysia’s recent times?
1. Kelantan has the highest number of AIDS cases
A model state with low crime rate, Boss Level morality, and always the frontliner in saying ‘NO’ to Valentine’s Day, PDA, etc. – that’s Kelantan. So it’s gonna come as a shock to you guys (or maybe not) that Kelantan’s vices are way over its head.
The state has consistently recorded the highest number of cases for AIDS in our country since 2008! Bbbut bbb but, they have like separate swimming lanes for men and women over there. How did the disease spread?!
Malaysian AIDS Council President Datuk Dr. Raj Karim explained that it’s partly due to the location of the state which borders Thailand. Sooo…
“Many men cross over to the neighbouring country, especially Sungai Golok and Hat Yai, without the knowledge of their wives, and then passed HIV and AIDS to them.” – Datuk Dr. Raj Karim, Malaysian AIDS Council, New Straits Times
And because of this, the women too are exposed to AIDS through their spouses and boyfriends, which have put them on top of the list compared to women in other states.
Image from Free Malaysia Today.
Malaysian AIDS Foundation Secretary-general Hisham Hussein said that HIV incidence there is 28.8 cases for every 100,000 in the population – DOUBLE Malaysia’s total incidence. Scary!
2. 6 AIDS experts lost their lives in the MH17 tragedy
This was United Nations AIDS Programme Director Michel Sidibe’s tweet expressing his condolences.
Technically this isn’t a statistic about the disease among Malaysians, but it relates to one of our biggest, most heartbreaking national tragedies. Among the passengers on the downed MH17 plane were big time AIDS researchers and activists who were flying to the International AIDS Conference in Melbourne. One of them was Professor Joep Lange from the Netherlands, who was known among his colleagues as ‘the father of AIDS research in developing countries.’
Joep’s work with HIV began when the virus emerged in the 1980s. His trial of anti-retroviral therapies has helped make the disease manageable. In fact, because of anti-retroviral drugs, nursing babies’ chances of contracting the virus have fallen to less than 1%.
Joep Lange. Image from The Guardian.
The world has lost :
-
Pim de Kuijer, Parliamentary lobbyist for Stop AIDS Now!
-
Lucie van Mens, Director of Support at The Female Health Company
-
Martine de Schutter, Programme Manager of Bridging the Gap
-
Glenn Thomas, World Health Organisation
-
Jacqueline van Tongeren, Amsterdam Institute for Global Health and Development
With about 50 countries in big ol’ Asia, our extended region is host to a large number of people living with AIDS/HIV. Although some countries within this region are seeing a decrease in the number of cases, our numbers are red, indicating an increase. According to a report by UNAIDS (2012), 12 countries account for more than 90% of people living with HIV and more than 90% new HIV infections in the Asia and the Pacific. As it turns out, Malaysia is ranked 8th in Asia and 5th in South East Asia.
However, because treatments are improving by the year, living with HIV or AIDS is no longer a death sentence but a manageable chronic condition. In efforts to eliminate the infection among children, our country is performing significantly better than most countries in our region. According to the CDC National Prevention Information Network (link goes to The Body, an HIV/AIDS site), 99 percent of babies born to HIV-infected women were uninfected by the virus. Women, Family, and Community Development Minister Daluk Rohani Abdul Karim attributes this to the success of our country’s Prevention of Mother-to-Child Transmission program which reaches out to pregnant women, their partners and infants.
4. Sharing needles isn’t the main cause of infections… It’s sex between men and women.
While the infection trend in Malaysia was predominantly from sharing of needles, the trend has changed in recent years. UNICEF shared that in 2010, there were 40% more newly-reported HIV cases attributed to heterosexual sex, which was a huge increase from 27% in the year before that. In a shocking report by The Star over the weekend, Professor Dr Adeeba Kamarulzaman, Chairman of the Malaysian AIDS Foundation, said that there were 74% new HIV infections in Malaysia that was sexually transmitted. She adds that it’s a drastic two-fold increase in just five years.
So what’s up? Perhaps the drop in IV transmission could be because of the Ministry of Health’s focus on drug substitution therapy and needle exchange programmes for drug users, which has been going on for a few years now.
“The alarming continued rise in new cases of sexually transmitted HIV – over 70% of all new cases in 2013 – warrants the immediate establishment and mobilisation of the proposed national task force for mitigation of sexual transmission of HIV.” – Datuk Dr. Raj Abdul Karim, President, Malaysian AIDS Council
5. Malaysia enforces pre-marital HIV testing for Muslim couples
On International AIDS Day 2001, the Johor Islamic Religious Council issued a fatwa that made HIV tests compulsory for any Muslim couples wanting to get married. This eventually became a nationwide thing in 2009 with recommendations for non-Muslims to do the same.
NAH, BACA:Here's how Dr M handled 4 super-awkward meetings with world leaders (and Trump)The effectiveness of this ruling in reducing the spread of HIV is still being put into question, with an equal number of supporters and detractors. This has also led to discussions of a person’s right to privacy, medical ethics, and follow-up support for those who have tested positive.
On the bright side though, there’s no nothing preventing the marriage from proceeding provided if the couple still wants to go ahead regardless; except additional counseling provided by the state religious and health departments.
6. More Malaysian women are being infected with HIV
In 2001, the ratio of HIV positive men to women was 10:1. Fast forward to 2013, this ratio is now 4:1, meaning that more women are being infected with the disease. Assuming that this isn’t due to better testing regulations (such as the mandatory HIV testing previously mentioned) which has led to more cases being detected, what would be the cause of the rise in HIV cases among women?
Loose morals? Prostitution?
You’d be surprised. The United Nations Children’s Fund (UNICEF) reports that for every sex worker testing positive for HIV, there are 13 housewives that do. Also, while men are most commonly infected through drug use, women are most commonly infected through heterosexual intercourse.
So wait, how are they getting infected then?
Well, for a number of reasons ranging from a lack of proper sex education to the submissive role of women in society. According to Jan Beagle, deputy executive director of UNAIDS, “It’s all linked ultimately to the economic and social empowerment of women” As quoted from Trust.org Many of the housewives infected with HIV are said to come from backgrounds where coerced sexual intercourse and strict obedience to husbands is the norm, even when it comes to condom use.
Because HIV/AIDS is commonly seen as a male oriented disease, women are usually neglected when it comes to studies (when was the last time you read anything about female drug users in Malaysia?), support, and regulation.
What we’ve strung together here are mainly numbers from reports and statistics, scratching just the surface of what could easily be an iceberg the size of Everest. One of the most important things which we didn’t tackle in this piece is the ignorance of the public towards the disease itself, which we think is the first step in doing our part to help those who live with this horrible disease.
Check out our freelancer Hanna Alkaf’s eye-opening article about some of the hurtful, horrible comments several Malaysians who live with HIV have had to endure here.
Because at the end, ignorance is not always bliss.
U.S. Naval War College -“Urban Outbreak 2019,” Pandemic Response
-