The shift from volume- to value-based care, delays in drug development (and the subsequent increase in development costs), growth in R&D spending, and support from regulatory bodies for the use of RWE solutions are some of the other major factors that are driving the growth of this market. However, the reluctance to rely on real-world studies and the lack of universally accepted methodological standards for data collection are restraining the growth of this market.
In 2020, the services segment accounted for a larger share of 58.8% of the global RWE solutions market. This segment is also estimated to grow at the highest CAGR of 16.0% during the forecast period. The rising need to convert data into actionable evidence, the growing need to reduce drug development delays, and the availability of a large volume of healthcare data are the major factors driving the growth of this market segment.
For More Information, Get Free Sample Report @ https://www.marketsandmarkets.com/requestsampleNew.asp?id=76173991
The emergence of this pandemic has posed severe financial constraints on pharma-biopharma companies in several countries. In this regard, RWE solutions have proven to be very helpful, as they allow industrial and academic researchers to monitor patients using digitally connected platforms while helping to organize and evaluate clinical data for regulatory submissions.
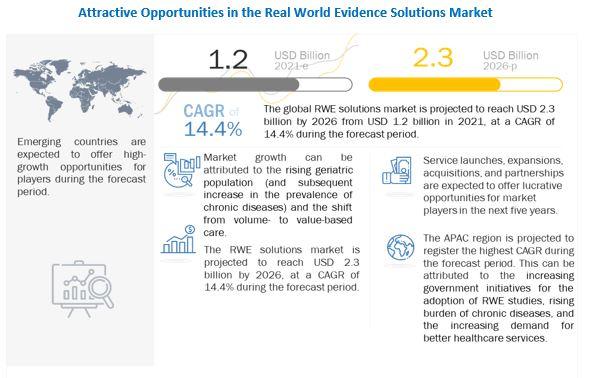
According to market research report, "Real World Evidence Market by Component (Dataset (Claims, Clinical, Pharmacy, Patient), Services), Therapeutic Area (Oncology, Cardiovascular, Neurology), End User (Pharma, Medtech, Payers, Providers) Covid-19 Impact - Global Forecast to 2026" The real world evidence solutions market size is projected to reach USD 2.3 billion by 2026, at a CAGR of 14.4% between 2021 and 2026 Real-world data and real-world evidence are used to monitor the post-market safety and adverse events of drugs. The monitoring of this data assists in making regulatory decisions.
