Hossein Moshiri

1. Introduction
Caustic soda, also known as sodium hydroxide (NaOH), is a versatile compound with numerous industrial applications, ranging from chemical manufacturing to water treatment and pulp and paper production. The traditional method of caustic soda production involves the use of mercury or diaphragm cells, which pose environmental and health risks. However, a more sustainable and efficient alternative has emerged in the form of bipolar electrolysis. This article provides a detailed description of the bipolar electrolysis process for caustic soda production, exploring its principles, equipment, electrolyte composition, electrochemical reactions, process control, and optimization. Additionally, it examines the advantages and challenges associated with this innovative technique. Understanding the bipolar electrolysis process is crucial for industries seeking to enhance their caustic soda manufacturing methods while prioritizing sustainability and safety.
1. Introduction to Bipolar Electrolysis
1.1 Importance of Caustic Soda in Industrial Processes
Caustic soda, also known as sodium hydroxide, is a chemical compound that plays a crucial role in various industrial processes. Its versatility makes it an essential ingredient in the production of a wide range of products, including paper, textiles, detergents, and even food. From manufacturing to wastewater treatment, caustic soda is a key component that enables countless industries to function efficiently and effectively.
1.2 Introduction to Bipolar Electrolysis as a Production Method
Bipolar electrolysis is a method used in the production of caustic soda, offering certain advantages over alternative techniques. Unlike the traditional diaphragm or membrane electrolysis processes, bipolar electrolysis eliminates the need for a separating barrier between the anode and cathode compartments. This simplifies the process, reduces maintenance, and improves overall efficiency. By understanding the principles and setup of bipolar electrolysis, we can grasp how this innovative technique revolutionizes caustic soda production.
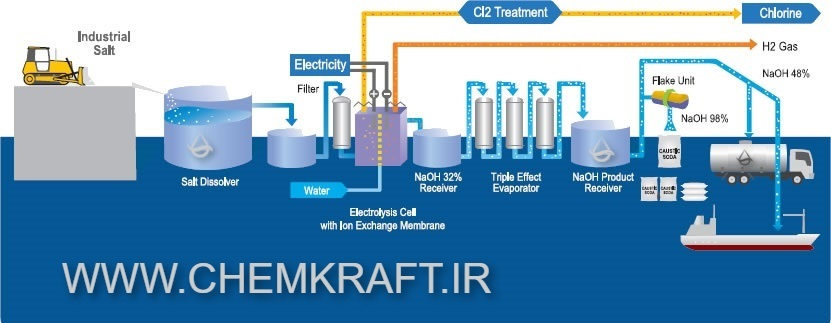
2. Overview of Caustic Soda and its Industrial Applications
2.1 Definition and Properties of Caustic Soda
Caustic soda, scientifically known as sodium hydroxide (NaOH), is a white, crystalline substance that is highly soluble in water. It is a strong alkali, capable of reacting with various substances, and has corrosive properties. Caustic soda is often found in solid form, either as flakes, pearls, or granules, and its concentration determines its strength in industrial applications.
2.2 Industrial Applications and Importance of Caustic Soda
Caustic soda is invaluable in a multitude of industrial applications. It serves as a powerful cleaning agent and is widely used in the production of detergents, soaps, and other cleaning products. The textile industry relies on caustic soda for processes such as mercerization, which enhances the strength and luster of fabrics. Additionally, caustic soda is involved in the production of pulp and paper, petroleum and gas refining, water treatment, and the manufacturing of various chemicals. Its versatility and importance make caustic soda a vital component in countless industrial processes.
3. Principles of Bipolar Electrolysis Process
3.1 Electrolysis as a Chemical Reaction
Electrolysis is a chemical reaction that occurs when an electric current is passed through a liquid or a solution containing ions. This process splits the compounds into their constituent elements. In the case of caustic soda production, electrolysis is employed to separate sodium (Na+) and hydroxide (OH-) ions from saltwater or brine solution, which is then used to produce caustic soda.
See also Investigating Potential Alternatives to Caustic Soda for Sustainable Glass Manufacturing
3.2 Understanding the Bipolar Electrolysis Technique
The bipolar electrolysis technique utilizes a unique setup where several bipolar electrodes are immersed in the electrolyte solution. These bipolar electrodes consist of alternating anode and cathode sections, enabling the simultaneous production of chlorine gas and caustic soda. The bipolar design eliminates the need for a physical barrier between the compartments, allowing for better ion transfer and improved efficiency. This innovative approach enhances the overall production process while reducing energy consumption and maintenance requirements.
4. Equipment and Setup for Bipolar Electrolysis Caustic Soda Production
4.1 Overview of Bipolar Electrolysis Plant
A bipolar electrolysis plant consists of various components designed to facilitate the caustic soda production process. These include electrolysis cells, bipolar electrodes, a power supply, brine storage tanks, and a production unit for caustic soda. The plant is carefully designed to ensure proper circulation of electrolyte solution, effective separation of chlorine gas and caustic soda, and efficient utilization of energy.
4.2 Components and Functions of Electrolysis Equipment
ٍlectrolysis equipment comprises of electrodes, cell compartments, and a power supply. The electrodes, made of materials such as titanium-coated steel, enable the conversion of electricity into chemical reactions. ژell compartments accommodate the bipolar electrodes and facilitate the separation of chlorine gas and caustic soda. The power supply provides the necessary electrical current to drive the electrolysis process.
4.3 Setup and Design Considerations
When setting up a bipolar electrolysis caustic soda production facility, several design considerations must be taken into account. These include optimizing the arrangement of bipolar electrodes, ensuring efficient circulation of brine solution, implementing safety measures for chlorine gas handling, and incorporating control systems to monitor and regulate the process parameters. Proper setup and design enable smooth operation, improve productivity, and ensure the safety of personnel and equipment.
5. Electrolyte Composition and Operating Conditions
5.1 Selection and Preparation of Electrolyte
In the bipolar electrolysis process for caustic soda production, the selection and preparation of electrolyte play a crucial role. The electrolyte used is typically a solution of sodium chloride (NaCl) in water. The concentration of the electrolyte solution is carefully controlled to optimize the efficiency and quality of the caustic soda production.
5.2 Optimal Operating Conditions for Bipolar Electrolysis
To ensure the successful production of caustic soda through bipolar electrolysis, specific operating conditions need to be maintained. These conditions include maintaining a suitable temperature range, controlling the current density, and ensuring proper circulation of the electrolyte solution. By carefully controlling these operating conditions, the efficiency and effectiveness of the electrolysis process can be maximized.
6. Electrochemical Reactions and Products in Bipolar Electrolysis
6.1 Anode and Cathode Reactions
During the bipolar electrolysis process, electrochemical reactions occur at both the anode and cathode. At the anode, chloride ions (Cl-) are oxidized to form chlorine gas (Cl2). Meanwhile, at the cathode, water molecules are reduced to produce hydrogen gas (H2) and hydroxide ions (OH-).
6.2 Formation of Sodium Hydroxide (NaOH) and Chlorine (Cl2)
The key products of bipolar electrolysis for caustic soda production are sodium hydroxide (NaOH) and chlorine gas (Cl2). The hydroxide ions (OH-) generated at the cathode combine with sodium ions (Na+) to form sodium hydroxide, which is a vital ingredient in various industrial applications. Simultaneously, chlorine gas is evolved at the anode and can be utilized in the production of bleach, PVC, and other chemicals.
7. Process Control and Optimization in Bipolar Electrolysis Caustic Soda Production
7.1 Monitoring and Controlling Electrolysis Parameters
To ensure the efficiency and quality of caustic soda production, meticulous monitoring and control of electrolysis parameters are essential. This involves closely monitoring variables such as temperature, current density, and electrolyte concentration. By carefully controlling these parameters, operators can optimize the production process and achieve desired results.
7.2 Strategies for Efficiency Enhancement and Product Quality
In the pursuit of improved efficiency and product quality in bipolar electrolysis, there are several strategies that can be employed. These may include optimizing the electrolyte composition, fine-tuning the operating conditions, and implementing advanced process control techniques. Additionally, continuous research and development efforts can further enhance the overall efficiency and product quality of the caustic soda manufacturing process.
8. Advantages and Challenges of Bipolar Electrolysis for Caustic Soda Manufacturing
8.1 Advantages of Bipolar Electrolysis over Conventional Methods
Bipolar electrolysis offers several advantages over conventional methods of caustic soda production. Firstly, it eliminates the need for a diaphragm or membrane, simplifying the overall process. Additionally, it allows for higher current densities, resulting in increased production rates. Moreover, bipolar electrolysis offers better control over the quality and purity of the caustic soda produced.
8.2 Challenges and Limitations of Bipolar Electrolysis Technique
While bipolar electrolysis has its advantages, it also presents certain challenges and limitations. One of the main challenges is the potential for increased energy consumption due to higher current densities. Additionally, maintaining the optimal operating conditions and ensuring uniformity throughout the electrolyte solution can be challenging. Moreover, the upfront investment required for implementing bipolar electrolysis technology may pose financial constraints for some manufacturers.
In conclusion, bipolar electrolysis offers a promising and sustainable approach to caustic soda production. By eliminating the need for mercury or diaphragm cells, this technique reduces environmental impact and improves overall safety. With the right equipment, electrolyte composition, and process control, industries can maximize efficiency and product quality while minimizing waste. However, challenges such as cost and operational complexities must be addressed to fully harness the potential of bipolar electrolysis. As advancements in technology continue, it is expected that this method will play a significant role in the future of caustic soda manufacturing, driving industry towards a greener and more efficient future.
See also Top 3 Caustic Soda Suppliers Based on Revenue
FAQ
1. What is caustic soda, and why is it important in industrial processes?
Caustic soda, also known as sodium hydroxide (NaOH), is a highly versatile compound used in various industrial applications. It is a strong alkaline substance that can be used for pH adjustment, chemical manufacturing, water treatment, soap production, and many other processes.
2. How does bipolar electrolysis differ from traditional methods of caustic soda production?
Bipolar electrolysis is a more sustainable and efficient alternative to traditional caustic soda production methods such as mercury or diaphragm cells. Unlike these methods, bipolar electrolysis eliminates the use of hazardous materials, making it safer for both workers and the environment. It also offers higher energy efficiency and better controllability, resulting in improved product quality.
3. What are the advantages of using bipolar electrolysis for caustic soda manufacturing?
Bipolar electrolysis offers several advantages over conventional methods. It reduces environmental impact by eliminating the need for mercury, which is a toxic substance, and minimizing waste generation. The process also allows for better energy efficiency, improved control over the electrolysis parameters, and enhanced product quality. Moreover, it aligns with sustainable manufacturing practices, making it an attractive option for industries.
4. Are there any challenges associated with bipolar electrolysis for caustic soda production?
While bipolar electrolysis shows great promise, there are some challenges that need to be addressed. One challenge is the initial investment required for implementing the necessary equipment and infrastructure. Additionally, the process requires careful control and monitoring to optimize electrolysis parameters for efficient and consistent production. Overcoming these challenges will be vital to fully realize the potential of bipolar electrolysis in caustic soda manufacturing.
Hossein Moshiri
WA +989124311007
