Increasing incidences of peripheral vascular diseases, liver cancer, strokes, and uterine fibroids coupled with rising preference for minimally invasive procedures; increasing investments, funds, and grants by public-private organizations for research; and technological advancements in the market will fuel the market growth over the forecast period.
The embolotherapy market is witnessing a loss of business, and the trend is expected to continue till December 2020. Unfavorable changes in regulations and guidelines are hampering the growth of this industry. Major regulatory authorities across the globe (such as CDC, WHO, MHRA, TGA, and EMA) have identified that cancer patients are at greater risk of COVID-19 infection than healthy adults.
The embolotherapy devices market has experienced steady growth over the last decade, owing to the growing target patient population base, which has increased the number of target surgeries performed.
For Any Query or Customization need to add in “Embolotherapy Market Report” Kindly Follow this URL: https://www.marketsandmarkets.com/requestCustomizationNew.asp?id=185897830
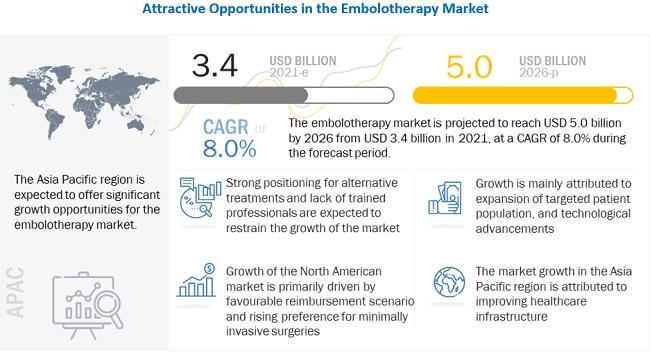
The embolotherapy devices market has experienced steady growth over the last decade, owing to the growing target patient population base, which has increased the number of target surgeries performed. CVD is a key target disease; its importance and high prevalence have made it a focus for research on successful and effective therapies.
The high success rate and less post-operative complication rate associated with embolotherapy procedures coupled with the rising incidences of liver cancer and hepatocellular cancer will further boost the demand for embolotherapy devices in the near future. Liver cancer is fifth-most-common cancer in men and the ninth-most-common in women.
To Know the Impact of COVID-19 on Embolotherapy Market: https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=185897830
The availability of effective conventional first-level therapies for the treatment of liver cancer, uterine fibroids, hemorrhagic stroke, and other hemorrhagic conditions is a key restraint to market growth. For instance, therapies such as surgery, chemotherapy, and radiation therapy are preferred options in cancer treatment due to the high awareness among individuals about traditional cancer treatments, low adoption of advanced treatment options (especially in developing countries), and the reluctance among oncologists to shift from conventional treatment options.
The applications of embolotherapy continue to expand. Initially, embolotherapy was applied for uncontrolled gastrointestinal bleeding; in recent years, embolotherapy is being increasingly adopted for various indications such as non-operative management of solid tumors of the liver, spleen, and kidney; visceral and solid organ aneurysm; vascular malformations in the central nervous systems, pulmonary circulation, head & neck, trunk, and extremities; uterine fibroids; and benign prostatic tumors.
Healthcare markets are witnessing a dearth of well-trained surgeons (including neurosurgeons and radiologists). The Association of American Medical Colleges (US) has estimated a deficit of 41,000 general surgeons in the US by 2025 (Source: Association of American Medical Colleges US, 2017). A shortage of more than 2,300 medical oncologists is expected in the US by 2025 (Source: Journal of Global Oncology).
Key Market Players
The major players in the embolotherapy market are Boston Medical Corporation (US), Terumo Medical Systems (Japan), Medtronic (US), Johnson & Johnson (US), and Stryker Corporation (US). Other key players in the embolization therapy market include Sirtex Medical Limited (US), Abbott Laboratories (US), Acandis GmbH (Germany), Balt (France), Cook Medical (US), Kaneka Corporation (Japan), Penumbra, Inc. (US), Merit Medical Systems (US), Meril Life Sciences Pvt. Ltd. (India), Cardiva (US), Shape Memory Medical Inc. (US), Artio Medical Inc. (US), Rapid Medical (US), Emboline, Inc. (US), and IMbiotechnologies Ltd. (Canada).
