In the world of chemistry, understanding the intricate processes that govern reactions and transformations is key to mastering the subject. Whether you're a budding scientist or a student navigating the depths of chemistry, a solid foundation is essential. This is where chemistry tuition plays a crucial role. In this post, we will delve into the fascinating topic of electrolysis in simple electric cells, exploring the fundamental principles, applications, and factors affecting this process. Along the way, we will highlight the benefits of enrolling in chemistry tuition to unlock the mysteries of chemistry effectively.
The Basics of Electrolysis
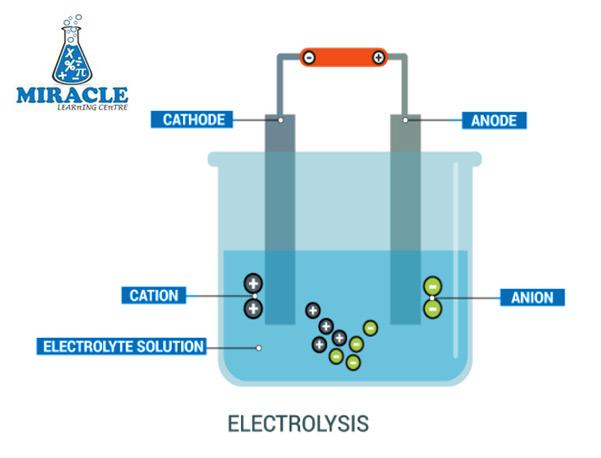
Electrolysis is a fundamental concept in chemistry that revolves around the breaking down of compounds into their constituent elements using an electric current. At its core, electrolysis involves the use of two electrodes immersed in an electrolyte solution. The electrodes, typically made of conductive materials like graphite or metal, facilitate the flow of electric charge through the solution. The electrolyte, in turn, contains ions that migrate towards the electrodes under the influence of the electric field.
Chemical Reactions During Electrolysis
During electrolysis, chemical reactions occur at the electrodes. At the cathode (the negatively charged electrode), reduction reactions take place, leading to the gain of electrons by cations (positively charged ions). Conversely, at the anode (the positively charged electrode), oxidation reactions occur, causing the loss of electrons by anions (negatively charged ions). These reactions ultimately result in the separation of the compounds into their constituent elements.
Factors Affecting Electrolysis
Factors affecting electrolysis are crucial in determining the rate and efficiency of the electrochemical process. These factors include:
- Voltage (Electrical Potential)
The voltage applied across the electrodes in an electrolytic cell directly influences the rate of electrolysis. Higher voltage leads to a greater flow of electric current and, consequently, faster electrolysis.
- Concentration of Electrolyte
The concentration of ions in the electrolyte solution impacts the conductivity and efficiency of electrolysis. A more concentrated electrolyte solution typically results in a higher rate of electrolysis.
- Temperature
Temperature affects the kinetic energy of molecules in the solution. Higher temperatures generally increase the mobility of ions, making them more readily available for the electrolysis process and thus accelerating it.
- Surface Area of Electrodes
The size and surface area of the electrodes in the cell can influence the rate of electrolysis. Larger electrodes provide more surface for reactions to occur, potentially increasing the rate of electrolysis.
- Type of Electrolyte
The specific type of electrolyte used can significantly affect the electrolysis process. Different electrolytes have varying conductivities and chemical properties, which can lead to differences in reaction rates during electrolysis.
- Presence of Catalysts
In some cases, catalysts are used to increase the rate of specific electrode reactions. Catalysts can reduce the activation energy required for a reaction to occur, thus accelerating the electrolysis process.
Applications of Electrolysis
It has a wide range of applications across various industries and fields. Some of the most common applications of electrolysis include:
- Electroplating:
Electrolysis is widely used for electroplating metals like gold, silver, nickel, and chromium onto other materials. This process is used in the manufacturing of jewellery, silverware, automotive parts, and various electronic components to improve their appearance and corrosion resistance.
- Water Electrolysis:
Electrolysis of water is used to produce hydrogen and oxygen gases. These gases have numerous applications, including fuel cells for clean energy generation, rocket propulsion, and as a source of pure gases in laboratories.
- Chlorine Production:
The electrolysis of brine (sodium chloride solution) produces chlorine gas, sodium hydroxide (caustic soda), and hydrogen gas. Chlorine is used in the production of various chemicals, including plastics and solvents, while sodium hydroxide is used in pulp and paper manufacturing and as a cleaning agent.
- Wastewater Treatment:
Electrocoagulation, a form of electrolysis, is used in wastewater treatment to remove contaminants and pollutants from water. The process involves the formation of metal hydroxide flocs that can adsorb impurities.
- Electrolysis in Electrochemical Cells:
Batteries and fuel cells are examples of electrochemical cells that use electrolysis principles to convert chemical energy into electrical energy. These cells have applications in powering electronic devices, vehicles, and more.
Importance of Safety in Electrolysis
When dealing with electrolysis, safety is paramount. Handling electric current, chemical reactions, and various apparatus can pose risks if not approached with caution. Chemistry tuition not only imparts knowledge about the process itself but also emphasizes the importance of safety protocols and precautions in the laboratory. Students learn to handle equipment and chemicals responsibly, reducing the risk of accidents.
Conclusion:
In conclusion, electrolysis in simple electric cells is just one of the many fascinating aspects of chemistry. To truly unlock the potential of this science and navigate its complexities, there's no substitute for chemistry tuition. It provides the guidance, support, and knowledge necessary to excel in chemistry studies, whether for academic purposes or a lifelong passion for science. So, if you're looking to master the principles of electrolysis and embark on a rewarding chemistry journey, consider enrolling in chemistry tuition. It's the surest way to electrify your understanding of this captivating subject.