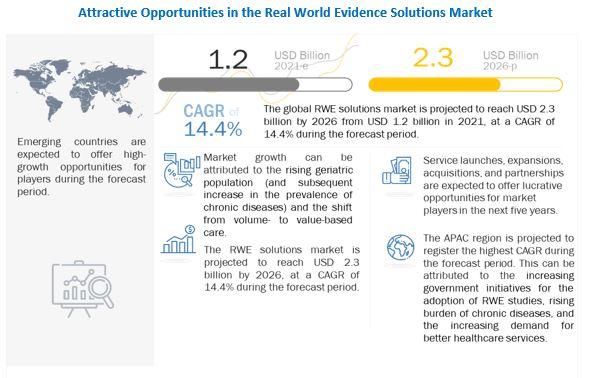
The real world evidence solutions market size is projected to reach USD 2.3 billion by 2026, at a CAGR of 14.4% between 2021 and 2026 Real-world data and real-world evidence are used to monitor the post-market safety and adverse events of drugs. The monitoring of this data assists in making regulatory decisions. The healthcare community is using RWE and RWD to support coverage decisions and to develop guidelines and decision support tools for use in clinical practice.
The rising geriatric population (and the subsequent increase in the prevalence of chronic diseases) is a key factor driving the growth of this market. The shift from volume- to value-based care, delays in drug development (and the subsequent increase in development costs), growth in R&D spending.
RWE solutions are some of the other major factors that are driving the growth of this market. However, the reluctance to rely on real-world studies and the lack of universally accepted methodological standards for data collection are restraining the growth of this market.
In this report, the RWE solutions market is segmented based on component, therapeutic area, end user, and region.
Download PDF Brochure @ https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=76173991
Even though emerging technologies, such as telemedicine, have existed for decades, most of healthcare systems rely heavily on in-person interactions between patients and clinicians. Nevertheless, the current requirement for social distancing measures is swiftly pushing the primary care provision toward remote care.
The utilization of RWE in infectious disease control is not a new concept. During the Ebola outbreak in 2014, forecasters successfully used Global Epidemic and Mobility (GLEaM) simulations that combined real-world data on populations and their mobility with rigorous stochastic models of disease transmission to predict the global spread of the disease.
In 2020, the services segment accounted for a larger share of 58.8% of the global RWE solutions market. This segment is also estimated to grow at the highest CAGR of 16.0% during the forecast period. The rising need to convert data into actionable evidence, the growing need to reduce drug development delays, and the availability of a large volume of healthcare data are the major factors driving the growth of this market segment.
The oncology segment accounted for the largest share of 24.8% of the RWE solutions market in 2020. This segment is projected to reach USD 560.5 million by 2026 from USD 285.5 million in 2021, at a CAGR of 14.4% during the forecast period. The large share of this segment can be attributed to the high number of clinical trials conducted for oncology and the rising prevalence of cancer worldwide.
Prominent players in the real world evidence solutions market include IQVIA (US), IBM Corporation (US), ICON plc. (Ireland), PAREXEL International Corporation (US), PPD, LLC (US), Optum, Inc. (US), Cognizant Technology Solutions Corporation (US), Oracle Corporation (US), SAS Institute Inc. (US), Syneos Health, Inc. (US), Anthem, Inc. (US), Clinigen Group plc. (UK), Medpace Holdings Inc. (US) and Flatiron Health, Inc. (US). These companies adopted strategies such as service launches, expansions, agreements, partnerships, collaborations, and acquisitions to strengthen their market presence in the real world evidence solutions market.
