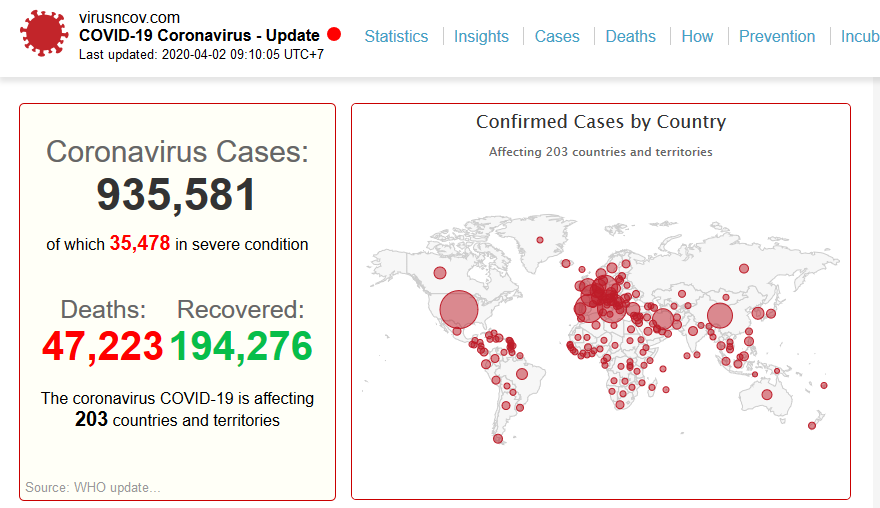
As more drastic measures are put in place around the world slow down the spread, scientists are attempting to find a treatment.
About 35 companies and academic institutions are racing to create a vaccine, and at least four already have candidates they have been testing in animals.
The first of these – produced by Boston-based biotech firm Moderna – will enter human trials imminently.
https://www.statnews.com/2016/09/13/moderna-therapeutics-biotech-mrna/
https://www.investors.com/news/technology/moderna-ipo-biotech/
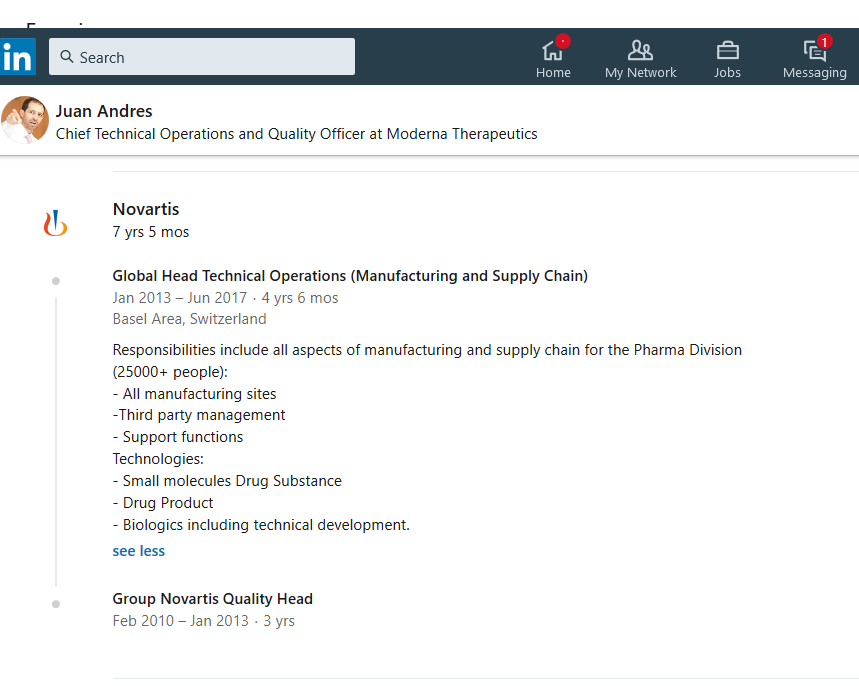
https://www.linkedin.com/in/juan-andres-65129b12/
Controversial Startup Moderna Just Filed for the Biggest Biotech IPO Ever
https://aws.amazon.com/solutions/case-studies/moderna-therapeutics/
https://www.cnbc.com/2018/12/10/cramer-dont-judge-biotech-moderna-on-its-stocks-post-ipo-drop.html
Don’t judge early-stage biotech Moderna on its stock’s post-IPO drop: Cramer
WATCH: Cramer introduces Moderna, newest biotech spec
https://fortune.com/2018/11/09/moderna-ipo-mrna/
29 Jan 2013
https://webcache.googleusercontent.com/search?q=cache:https://www.modernatx.com/pipeline
Moderna’s mRNA-1273
The Coalition for Epidemic Preparedness Innovations (CEPI) launched today at the World Economic Forum in Davos, Switzerland, with $460 million in funding
http://www.cidrap.umn.edu/news-perspective/2017/01/new-460-million-effort-takes-aim-mers-lassa-nipah
http://www.cidrap.umn.edu/infectious-disease-topics/covid-19
https://www.weforum.org/agenda/2020/04/covid19-furlough-employers-workers-support-global/
https://smw.ch/article/doi/smw.2020.20203
https://twitter.com/WuhanAnalysis/status/1226276257651986432
https://twitter.com/AdamJKucharski/status/1244687442885689345
https://cmmid.github.io/topics/covid19/severity/global_cfr_estimates.html
+++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++
Trump has claimed hydroxychloroquine showed “tremendous promise” in regards to treating the COVID-19 strain of coronavirus.
saying: “It is known as a malaria drug, and it’s been around for a long time and it’s very powerful.
“But the nice part is, it’s been around for a long time, so we know that if things don’t go as planned, it’s not going to kill anybody.”
hydroxychloroquine
SEPT 2016 biotech’s most secretive startups
https://statnews.com/2016/09/13/moderna-therapeutics-biotech-mrna/…
Dec 2018 Record Biotech IPO
Moderna Therapeutics Sets Record for Biggest Biotech IPO
Published: Dec 07, 2018 By Mark Terry

The long-awaited and massively hyped initial public offering (IPO) of Moderna Therapeutics hit the market yesterday. The company sold approximately 26.3 million shares priced at $23 a share. This exceeded the revised goal of $600 million by about $4.3 million. Shares are trading on the Nasdaq under the “MRNA” ticker symbol.
The raise values the company at about $7.5 billion.
raised $324 million at a $2.2 billion market cap. Other top biotech IPOs included Axovant Sciences, which raised $315 million in 2015, giving it a $1.5 billion initial market cap, and Galapagos NV, which raised $275 million in an IPO in 2015, with an initial market cap of $1.7 billion. And a year before them, in 2014, Juno Therapeutics raised $264 million in its IPO, with a $2.2 billion market cap. Celgene acquired Juno earlier this year for $9 billion.
Last week Moderna refiled with the Securities and Exchange Commission (SEC), raising its goal for its initial public offering from $500 million to $600 million. The company, which has no products on the market, is fantastic at raising money, but some analysts wonder if the company is overvalued. Since its founding in 2010, Moderna has raised more than $2.6 billion in equity financing. As of September 30, it had cash, cash equivalents, and investments of $1.2 billion.
The company focuses on messenger RNA (mRNA) therapeutics. mRNA’s role is to transport genetic information from DNA to the ribosome, offering up the amino acid sequence of the eventual proteins the DNA is coding for. Moderna’s tech platform is designed to engineer mRNA to deliver whatever protein codes they want the cells to produce, in effect, turning the cells themselves into vaccine or drug-manufacturing factories.
Moderna has a development pipeline of 21 programs. Ten are in the clinic and another three have open Investigational New Drug (INDs) submissions. Nine of those in the clinic are in Phase I and one is in Phase II, according to a July corporate update.
Although there is some skepticism as to whether Moderna can live up to expectations, it is unusual in the size of its pipeline. Most biotechs either have no products in the clinic when they launch an IPO, or they have one or two in early-stage trials and the funding is largely designed to help advance them into the clinic or more expensive late-stage trials.
Moderna’s chief financial officer, Lorence Kim, told Xconomy earlier this year, “We’re saying we can draw a picture that articulates an outsized return over time, and that outsized return comes from not one drug singly advancing by itself to approval, but instead by a technology that is pushed forward over time. The key thing for investors to wrap their arms around is: can we offer that sort of upside? We believe we can.”
Not unusual in biotech IPOs, Moderna is probably years away from having a product on the market. Meanwhile, it has pretty high overhead with 680 staffers and a highly-compensated executive suite. Stephen Hoge, Moderna president, in 2017 received $19 million in options and a $4.4 million cash bonus. Kim received $5.5 million in stock and a $1 million cash bonus. And company chief executive officer Stephane Bancel received $4.6 million in options and a $1.5 million cash bonus. Those three accounted for a combined $40 million in cash and stock last year.
And in its latest SEC filings, it indicated it burned through almost $360 million in operating expenses in the first nine months of this year. It also stated it had accumulated a deficit of $865.2 million.
Bancel owned 10 percent of the company ahead of the IPO. He’s the second-largest shareholder, behind Flagship Pioneering, which owned 19.5 percent prior to the IPO. AstraZeneca was the third-largest investor, with a pre-IPO stake of 8.4 percent.
The company has been secretive over its history, which it could be because it was privately owned. Now that it has gone public, it will face far more intense scrutiny. As most analysts note, and the company stated in its IPO filings, it’s not clear if mRNA drug processes works or are safe. In addition, regulatory agencies such as the U.S. Food and Drug Administration (FDA) haven’t evaluated these types of medicines, which makes the regulatory pathway uncertain.
https://biospace.com/article/moderna-therapeutics-biggest-ipo-in-biotech-
2020 #Funding Award from
Moderna Announces Funding Award from CEPI to Accelerate Development of Messenger RNA (mRNA) Vaccine Against Novel Coronavirus
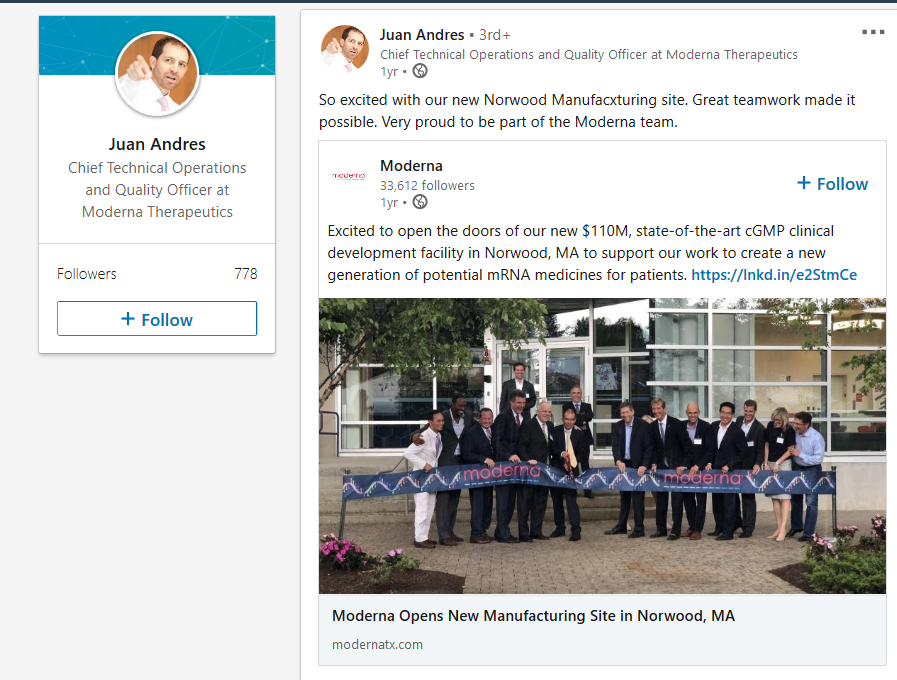
Moderna Announces Funding Award from CEPI to Accelerate Development of Messenger RNA (mRNA) Vaccine Against Novel Coronavirus Collaboration includes the National Institutes of Health (NIH) and leverages flexibility of Moderna’s mRNA vaccine technology Business Wire CAMBRIDGE, Mass. -- January 23, 2020 Moderna, Inc., (Nasdaq: MRNA) a clinical stage biotechnology company pioneering messenger RNA (mRNA) therapeutics and vaccines to create a new generation of transformative medicines for patients, and the Coalition for Epidemic Preparedness Innovations (CEPI), today announced a new collaboration to develop an mRNA vaccine against the novel coronavirus (2019-nCoV). Under the terms of the agreement, Moderna will manufacture an mRNA vaccine against 2019-nCoV, which will be funded by CEPI. The Vaccine Research Center (VRC) of the National Institute of Allergy and Infectious Diseases (NIAID), part of NIH, collaborated with Moderna to design the vaccine. NIAID will conduct IND-enabling studies and a Phase 1 clinical study in the U.S. Over the past four years Moderna has had six positive Phase 1 clinical readouts in its prophylactic vaccines modality and moved two additional programs into development. Moderna’s technology platform, fully integrated manufacturing site and development experience, combined with a multi-year relationship with the NIH, including exploring ways to respond to public health threats, allows for the rapid identification and advancement of a vaccine candidate against 2019-nCoV. “Moderna’s commitment to global public health is aligned with CEPI’s vision of creating a world in which epidemics are no longer a threat to humanity,” said Richard Hatchett, M.D., CEO of CEPI. “We are pleased with the pace of our combined response to the emerging threat of the novel coronavirus. Through our partnership with Moderna and the NIH, we hope to speed the development of a vaccine against the coronavirus and help to alleviate the burden of disease.” “We believe our mRNA vaccine technology offers potential advantages in the speed of development and production scalability, which positions Moderna to potentially develop a vaccine against coronavirus, 2019-nCoV,” said Stéphane Bancel, CEO of Moderna. “Advances in global public health require the collective effort of public-private partnerships – no organization can act alone. We are honored to be supporting NIH and CEPI in their mission to identify a potential vaccine to prevent infection. It is impressive that CEPI was able to commit to this grant in a matter of days. We are thankful for the financial support from CEPI and the multi-year scientific collaboration we have with the NIH.” About Coronavirus Coronaviruses are a family of viruses that can lead to respiratory illness, including Middle East Respiratory Syndrome (MERS-CoV) and Severe Acute Respiratory Syndrome (SARS-CoV). Coronaviruses are transmitted between animals and people and can evolve into strains not previously identified in humans. On January 7, 2020, a novel coronavirus (2019-nCoV) was identified as the cause of pneumonia cases in Wuhan City, Hubei Province of China, and additional cases have been found in a growing number of countries.^1,2 About Moderna’s Prophylactic Vaccines Modality Moderna scientists designed the Company’s prophylactic vaccines modality to prevent or control infectious diseases. This modality now includes five programs, all of which are vaccines against viruses. More than 1,000 participants have been enrolled in Moderna’s infectious disease vaccine clinical studies under health authorities in the U.S., Europe and Australia. The potential advantages of an mRNA approach to prophylactic vaccines include the ability to mimic natural infection to stimulate a more potent immune response, combining multiple mRNAs into a single vaccine, rapid discovery to respond to emerging pandemic threats and manufacturing agility derived from the platform nature of mRNA vaccine design and production. Moderna currently has five development candidates for potential commercial uses in this modality, including: respiratory syncytial virus (RSV) vaccine (mRNA-1777 and mRNA-1172 or V172 with Merck), cytomegalovirus (CMV) vaccine (mRNA-1647), human metapneumovirus and parainfluenza virus type 3 (hMPV/PIV3) vaccine (mRNA-1653) and Zika vaccine (mRNA-1893) with the Biomedical Advanced Research and Development Authority (BARDA). Three development candidates in this modality are available for potential global health uses including: influenza H10N8 vaccine (mRNA-1440), influenza H7N9 vaccine (mRNA-1851) and chikungunya vaccine (mRNA-1388), which was developed with the Defense Advanced Research Projects Agency (DARPA). To date, Moderna has demonstrated positive Phase 1 data readouts for six prophylactic vaccines (H10N8, H7N9, RSV, chikungunya virus, hMPV/PIV3 and CMV). Moderna’s CMV vaccine is currently in a Phase 2 dose-selection study. Moderna’s investigational Zika vaccine (mRNA-1893), currently in a Phase 1 study, was granted FDA Fast Track designation. Moderna has built a fully integrated, highly digitalized manufacturing plant in Norwood, MA which enables the promise of the technology platform.
April 2020 $483 mil USGOV
ULY 20 $472 MIL USGO
Moderna gets further $472 million US award for coronavirus vaccine development
thejakartapost.com The Jakarta Post
Diterbitkan : 12.54, 27/07/2020

A woman cleans the entrance doors to Moderna headquarters in Cambridge, Massachusetts on May 18, 2020.Moderna Inc said on Sunday it has received an additional $472 million from the US government's Biomedical Advanced Research and Development Authority (BARDA) to support development of its novel coronavirus vaccine.
Moderna Inc said on Sunday it has received an additional $472 million from the US government's Biomedical Advanced Research and Development Authority (BARDA) to support development of its novel coronavirus vaccine.
The US-based drug maker said the additional funding will support its late-stage clinical development including the expanded Phase 3 study of Moderna's vaccine candidate.
In April, Moderna had received $483 million from the US federal agency that funds disease-fighting technology, when the experimental vaccine was in an early-stage trial conducted by the US National Institutes of Health.
"Encouraged by the Phase 1 data, we believe that our mRNA vaccine may aid in addressing the COVID-19 pandemic and preventing future outbreaks," Chief Executive Officer Stéphane Bancel said in a press release.
BARDA's total funding for the experimental vaccine of Moderna, the first in the United States to begin human trials of a coronavirus vaccine, is now about $955 million.
The vaccine uses synthetic messenger RNA (mRNA) to inoculate against the coronavirus. Such treatments help the body immunize against a virus and can potentially be developed and manufactured more quickly than traditional vaccines.
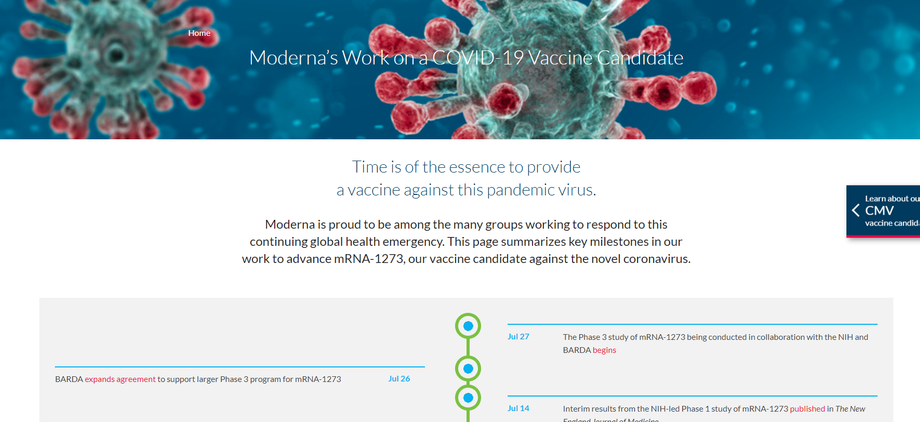
A Phase 3 study, conducted in collaboration with the National Institute of Allergy and Infectious Diseases, will begin on July 27 and involve about 30,000 participants, according to the company.
Moderna said it remains on track to be able to deliver about 500 million doses per year, and possibly up to 1 billion doses per year, beginning in 2021.
The announcement about further funding came two days after the drug developer said its formula used in developing the vaccine was not covered under patents owned by Arbutus Biopharma
Pfizer Inc, Novavax Inc, Britian's AstraZeneca Plc are other few drug makers that received funding from BARDA for coronavirus vaccine development.
SO WTF
Several more studies have confirmed the effectiveness
On 22 May the Lancet published a blockbuster peer-reviewed study which found the antimalarial drug hydroxychloroquine, which has been promoted by Donald Trump, was associated with a higher mortality rate in Covid-19 patients and increased heart problems.
Trump, much to the dismay of the scientific community, had publicly touted hydroxychloroquine as a “wonder drug” despite no evidence of its efficacy for treating Covid-19.
The Lancet study, which listed Desai as one of the co-authors, claimed to have analysed Surgisphere data collected from nearly 96,000 patients with Covid-19, admitted to 671 hospitals from their database of 1,200 hospitals around the world, who received hydroxychloroquine alone or in combination with antibiotics.
The negative findings made global news and prompted the WHO to halt the hydroxychloroquine arm of its global trials.
But only days later Guardian Australia revealed glaring errors in the Australian data included in the study. The study said researchers gained access to data through Surgisphere from five hospitals, recording 600 Australian Covid-19 patients and 73 Australian deaths as of 21 April.
But data from Johns Hopkins University shows only 67 deaths from Covid-19 had been recorded in Australia by 21 April. The number did not rise to 73 until 23 April. Desai said one Asian hospital had accidentally been included in the Australian data, leading to an overestimate of cases there. The Lancet published a small retraction related to the Australian findings after the Guardian’s story, its only amendment to the study so far.
The Guardian has since contacted five hospitals in Melbourne and two in Sydney, whose cooperation would have been essential for the Australian patient numbers in the database to be reached. All denied any role in such a database, and said they had never heard of Surgisphere. Desai did not respond to requests to comment on their statements.
Another study using the Surgisphere database, again co-authored by Desai, found the anti-parasite drug ivermectin reduced death rates in severely ill Covid-19 patients. It was published online in the Social Science Research Network e-library, before peer-review or publication in a medical journal, and prompted the Peruvian government to add ivermectin to its national Covid-19 therapeutic guidelines.
The New England Journal of Medicine also published a peer-reviewed Desai study based on Surgisphere data, which included data from Covid-19 patients from 169 hospitals in 11 countries in Asia, Europe and North America. It found common heart medications known as angiotensin-converting–enzyme inhibitors and angiotensin-receptor blockers were not associated with a higher risk of harm in Covid-19 patients.
BOTH NOW RETRACTED YET FDA HAVING REVERSED ITS DECISION ALLOWING ITS USE OFF LABEL FOR COV19 HAS NOT REVERSED IT DECISION
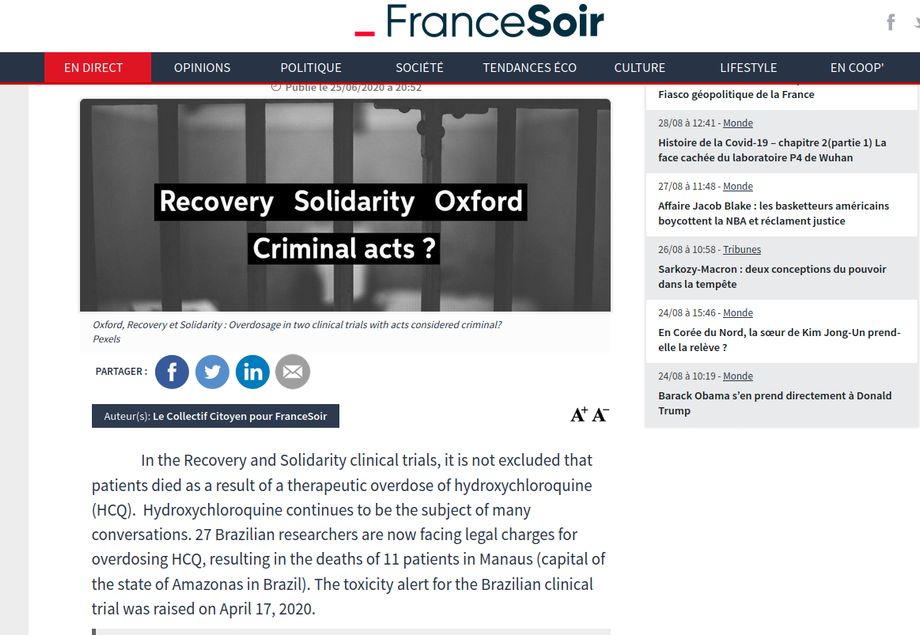
In the Recovery and Solidarity clinical trials, it is not excluded that patients died as a result of a therapeutic overdose of hydroxychloroquine (HCQ). Hydroxychloroquine continues to be the subject of many conversations. 27 Brazilian researchers are now facing legal charges for overdosing HCQ, resulting in the deaths of 11 patients in Manaus (capital of the state of Amazonas in Brazil). The toxicity alert for the Brazilian clinical trial was raised on April 17, 2020.
A synthetic COVID virus highly pathogenic for humans created by the Wuhan Institute of Virology and the USA.

TRIBUNE ANALYSIS- This is not yet another fake news but an indisputable reality described in an article freely available on the internet, published in 2015 in Nature Medicine. A completely extraordinary scientific text which describes the manufacture of a viable chimeric synthetic COVID virus and highly pathogenic for humans on the pretext of studying the pandemic potential of coronaviruses of animal origin of which bats are a natural reservoir .
Editor's note: this article represents an analysis of coronaviruses in general which are not to be confused with Covid-19 which is a subset of it. According to an article in Nature, SARS-Cov2 would probably be associated as a Nature article says with the bat. (updated July 20, 2020)
The scientists responsible for this research are Ralph S. Baric , professor and director of the microbiology laboratory at Chapel Hill University (North Carolina) and Shi Zheng Li , doctor in microbiology graduated from the Faculty of Sciences of Montpellier, now at head of the biosafety and special pathogens laboratory at the famous Wuhan Institute of Virology.
In retrospect, this article takes on a premonitory character which questions the situation in which the entire planet was plunged in 2020.
After an introduction which mentions the global chaos which would result from a pandemic caused by a coronavirus of bats which would have crossed the species barrier to infect humans, we discover the detailed description of the manufacture of an artificial virus with a pathogenic character as marked as that of the 2002-2003 epidemic. This chimeric virus was a synthetic hybrid, constructed from the 2002-2003 mouse-adapted human acute respiratory syndrome (SARS) virus (SARS-MA15). In a second step, the surface protein S (spicule) of the virus adapted to mice was substituted by the spike protein SHC014 originating froma bat virus identified at the Wuhan institute. The presence of the SHC014 protein in a bat coronavirus that binds to ACE2 cell penetration receptors found in many mammals is the great discovery of Shi Zheng Li published in Nature in 2013.
The question was whether the SHC014 protein could activate human ACE2 directly without adaptation, since it had five mutations in regions essential for its binding to the ACE2 receptor compared to the S protein of SARS-Cov from 2002-2003. The chimeric virus produced, dubbed SHC014-MA15, was perfectly viable and replicated in human upper respiratory cell cultures at the same concentration levels as seen in patients of the deadly 2002-2003 epidemic. In contrast, laboratory mice infected with the hybrid SHC014-MA15 virus developed a noticeable pathogenic effect with weight loss but little mortality while those infected with the adapted SARS-MA15 virus died within 4 days.
Remarkably, the SHC014 protein conferred on the hybrid virus SHC014-MA15 a very pronounced infectious character and specific to the cells of the human upper respiratory tract without the need for adaptation mutations.
The surface protein SHC014 of the eponymous bat virus is therefore likely, once artificially integrated into other viruses, to enhance their pathogenesis to a potentially fatal level.comparable to that of the 2003 epidemic. This kind of research which consists in increasing the biological capacities of an organism by genetic manipulation obtained by microbiology is now banned from institutional research in the USA. Note that in his statements to the press a French virologist expert denied that such manipulations could have taken place in Wuhan or elsewhere. However, what about research in secret military laboratories? On reading this article, we understand better why the Americans and Chinese accuse each other of having been at the origin of the SARS-Cov2 (COVID-19) pandemic ...
We also better understand the fears that the authorities have about the feasibility of a vaccine , the perhaps limited duration of acquired immunity, but also the possibility of life-saving cross-immunity between coronaviruses. All these questions are dealt with in depth by the authors through a series of experiments carried out on their laboratory mice.
Valère Lounnas is a former scientific researcher at the European Molecular Biology Laboratory (EMBL) in Heidelberg. His specialty was the theoretical study of proteins and their function. He then worked in the pharmaceutical industry in computer-aided drug design (CMAO) before becoming a medical writer.
We are making available to interested readers the rigorous literal translation of this article published in 2015 in Nature Medecine.
Translation of the Nature Medecine article: Download the PDF version
FILE [A synthetic COVID virus highly pathogenic for humans created by the Wuhan Institute of Virology and the USA]
"
Construction of SARS-like chimeric viruses.
Both wild-type and chimeric viruses were derived from either SARS-CoV Urbani or the corresponding mouse-adapted (SARS-CoV MA15) infectious clone (ic) as previously described27. Plasmids containing spike sequences for SHC014 were extracted by restriction digest and ligated into the E and F plasmid of the MA15 infectious clone. The clone was designed and purchased from Bio Basic as six contiguous cDNAs using published sequences flanked by unique class II restriction endonuclease sites (BglI). Thereafter, plasmids containing wild-type, chimeric SARS-CoV and SHC014-CoV genome fragments were amplified, excised, ligated and purified. In vitro transcription reactions were then preformed to synthesize full-length genomic RNA, which was transfected into Vero E6 cells as previously described2. The medium from transfected cells was harvested and served as seed stocks for subsequent experiments. Chimeric and full-length viruses were confirmed by sequence analysis before use in these studies. Synthetic construction of chimeric mutant and full-length SHC014-CoV was approved by the University of North Carolina Institutional Biosafety Committee and the Dual Use Research of Concern committee.
https://www.nature.com/articles/nm.3985
Affiliations
BELOW IS CIA I THINK - -- DUMB ENOUGH
WOULD ANY ONE OTHER THAN AN AMERICAN USE CNN AS A SOURCE
OH LOOK CHINAS OPEN
 WHAT WAS THAT ABOUT NOT OPEN
WHAT WAS THAT ABOUT NOT OPEN
Case 2:20-cv-01277-JJT Document 1 Filed 06/29/20
file name -- ican-v-fda